As H5N1 bird flu continues to evolve and cross species, researchers reveal how the latest vaccine technologies, nanomedicine, and AI could shape the future of pandemic defense.
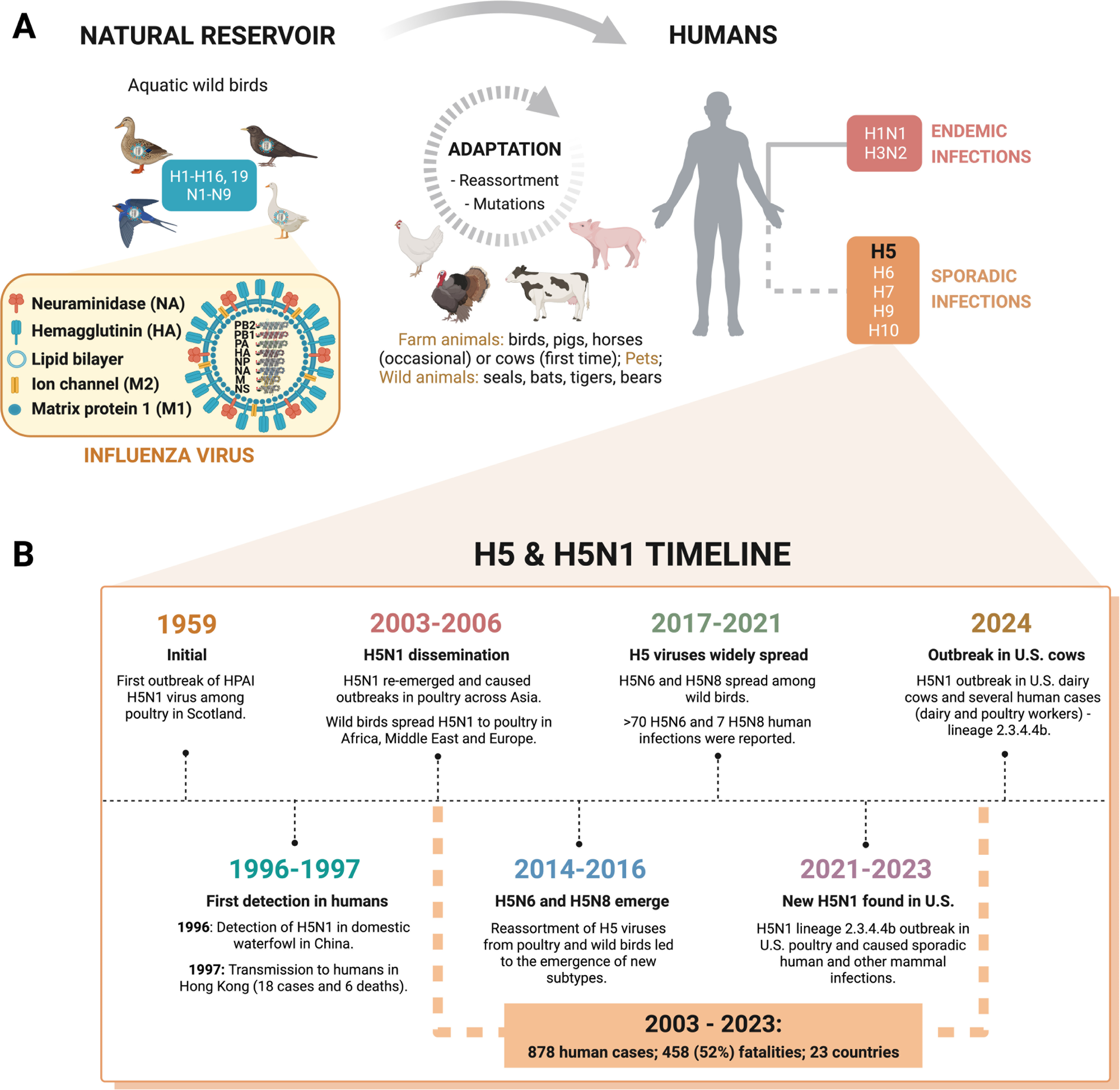
Influenza A virus diversity & host spillover. (A) IAV transmission. IAV jumps from its natural reservoir to different animals, including farmed animals, undergoing adaptation to this new biological environment. The virus can infect humans sporadically or acquire human-to-human transmission traits and become endemic such as the currently circulating subtypes H1N1 and H3N2 that cause yearly epidemics. (B) H5 and H5N1 timeline. HPAI H5N1’s first outbreak was reported in 1959 and first detected in humans in 1997, leading to 6 deaths. Since then, H5N1 and other emergent subtypes (e.g., H5N6 and H5N8) have caused several outbreaks with many human fatalities. Recently, the new H5N1 clade 2.3.4.4b caused an outbreak in U.S. dairy cows, subsequently transmitted to humans (dairy farm workers), threatening human health. Created with Biorender.
The global health landscape has been starkly reminded of the pandemic risk associated with zoonotic viruses with multiple host species. In a recent PEARLS article, a concise and educational overview published in the journal PLoS Pathogens, the authors explored how emerging influenza A viruses, particularly the dangerous hemagglutinin 5 neuraminidase 1 (H5N1) subtype, evolve and evade treatments and new cutting-edge strategies that could help control future outbreaks.
Influenza A virus: Zoonotic risk and evolution
Influenza A viruses (IAVs) are constantly evolving and pose a unique pandemic threat because they can spill over from various animal species to humans. Unlike seasonal flu strains, some subtypes, such as H5N1, originate in birds and have caused deadly outbreaks in other mammals. Recent reports detail H5N1 transmission in a wide range of animals, including cattle, with evidence of cattle-to-cattle and subsequent human spillover, resulting in 1 death among 70 confirmed human cases. The overall case fatality rate for H5N1 infections is approximately 52%.
Furthermore, although vaccines and antiviral drugs are available, they often fall short due to the virus’s rapid mutation and reassortment, which allow it to evade immune defenses and treatments. Additionally, high genetic diversity, especially in the virus's surface proteins, complicates vaccine design, limiting the use of current vaccines to target specific strains. Candidate vaccine viruses (CVVs) for all H5 subtypes are stockpiled, helping to shorten response times to outbreaks. However, H5N1 vaccines have shown poor immunogenicity in trials, even with adjuvants. Antiviral resistance is also growing, making it harder to manage infections and highlighting the need for more innovative strategies to combat IAV infections. The PEARLS article also notes that older individuals may retain partial immunity to H5N1 due to previous exposure to seasonal H1N1 or H2N2 viruses during childhood.
Current challenges and control strategies
In this educational overview, the authors conducted a comprehensive review to evaluate the current and emerging strategies for preventing and treating IAV infections, particularly those caused by the H5N1 subtype. They examined the diversity and host range of IAVs, focusing on the virus’s segmented ribonucleic acid (RNA) genome that facilitates mutation and genetic reassortment and allows IAVs to adapt quickly and infect new species, including humans.
The article then analyzed the development and limitations of current vaccines. It described how candidate vaccine viruses are selected based on circulating strains through global surveillance systems, with updates triggered by a four-fold or greater drop in neutralizing antibody titers against prevalent strains. The authors also discussed different vaccine platforms, including traditional egg- and cell-based production methods and newer messenger RNA (mRNA)-lipid nanoparticle (LNP) technologies.
Additionally, the PEARLS article reviewed the three main classes of antiviral drugs approved by regulatory agencies: M2 ion channel blockers, neuraminidase inhibitors, and viral polymerase inhibitors. M2 ion channel blockers (amantadine) are no longer recommended for monotherapy due to drug resistance, but recent in vitro data suggest some activity against H5N1 clade 2.3.4.4b. Various studies included in the review also evaluated the effectiveness of these drugs against the H5N1 clade 2.3.4.4b using in vitro and in vivo data. Baloxavir and favipiravir have demonstrated in vitro and in vivo efficacy against H5N1 clade 2.3.4.4b.
Moreover, the article examined numerous small molecules, peptides, and monoclonal antibodies being tested in clinical trials to explore future antiviral therapies. It also examined recent discoveries in viral biology, such as viral inclusions, which are membraneless structures exploited by the virus during the infection. It proposed targeting their physical properties to inhibit viral replication. The article notes that the liquidity of these inclusions is essential for viral replication and that hardening these condensates can suppress influenza A virus replication in vivo, representing a novel antiviral strategy.
Innovations and future directions
The PEARLS article reported that emerging strains of influenza A, particularly H5N1 clade 2.3.4.4b, are evolving rapidly, presenting serious challenges to current prevention and treatment strategies. However, several promising developments across vaccines, antiviral drugs, and surveillance technologies offer hope for improved control of future outbreaks.
Specifically, while traditional vaccines are strain-specific and often slow to adapt, newer mRNA-based vaccines have demonstrated strong immune responses in early clinical trials against potentially pandemic-causing strains such as H5N1. However, the article specifies that robust immune responses have been reported in phase I trials for mRNA vaccines targeting H10N8 and H7N9, while H5N1-specific candidates are still in preclinical testing. These vaccines could possibly overcome the weak immune responses seen with older formulations.
Furthermore, among antiviral drugs, existing neuraminidase inhibitors, such as oseltamivir, and polymerase inhibitors like baloxavir still show activity against the latest H5N1 strains. Recent in vitro studies show that H5N1 clade 2.3.4.4b viruses are susceptible to these drugs, and even to M2 ion channel blockers, although the latter are not currently recommended for use due to widespread resistance. Favipiravir, another polymerase inhibitor, also shows in vitro and in vivo efficacy. Some older drugs like the M2 ion channel blocker amantadine, although no longer recommended due to resistance, may also still work in specific cases.
Moreover, new drug candidates, including monoclonal antibodies and small molecules like DAS181 and nitazoxanide, are in various stages of clinical development and show promise against resistant virus strains.
One of the most promising insights from the article involves targeting viral inclusions, biomolecular condensates that the virus uses to replicate. Changing the physical properties of these compartments was shown to suppress viral replication. The article highlights that these membraneless condensates rely on “liquidity,” and “hardening” them inhibits replication in vivo. This mechanistic detail further supports this potentially new class of antiviral strategies.
However, the article also pointed out that drug testing models need improvement. Current animal model systems often fail to capture the complexity of human tissues. The widespread adoption of advanced models, such as organ-on-chip and other human-relevant systems, is still limited, and most preclinical animal models do not fully replicate human disease or transmission. Technologies like organ-on-chip systems and artificial intelligence (AI)-based drug discovery tools like AlphaFold are expected to revolutionize the identification and testing of new antivirals. AI tools not only accelerate molecule design but can also predict resistance and optimize drug target selection.
Nanotechnology could also enhance treatment delivery and efficacy, with some nanoparticles already showing the ability to reduce viral load and inflammation in animal studies. While the article discusses metal and metal-oxide nanoparticles (such as gold, silver, zinc oxide, zirconia) as broad-spectrum agents against influenza A viruses, animal model evidence for efficacy against H5N1 is mostly shown for zirconia (ZrO₂) and selenium nanoparticles. Nanoparticles can also be combined with conventional antivirals to enhance efficacy. However, the authors acknowledged that the virus’s unpredictable evolution makes it challenging to design universal solutions.
Conclusions
Overall, the PEARLS article underscored the urgent need to rethink our strategies against influenza A viruses. While new vaccines, antiviral drugs, and technological tools offer promising paths forward, the fast-paced evolution of viruses like H5N1 continues to outpace many existing approaches. The findings suggested that innovative solutions, ranging from AI-assisted drug discovery to nanomedicine, are crucial for improving preparedness and reducing the global threat posed by future influenza outbreaks.