The immune system relies on B cells and their ability to make antibodies against an extremely broad range of pathogens. This broad responsiveness bears some risk, as B cells can also turn against healthy tissue - a phenomenon called autoimmunity. Scientists from the lab of Meinrad Busslinger now reported in the journal “Nature Immunology” how the protein Ikaros orchestrates the fine balance between B cell silencing and activation - and thereby controls autoimmunity.
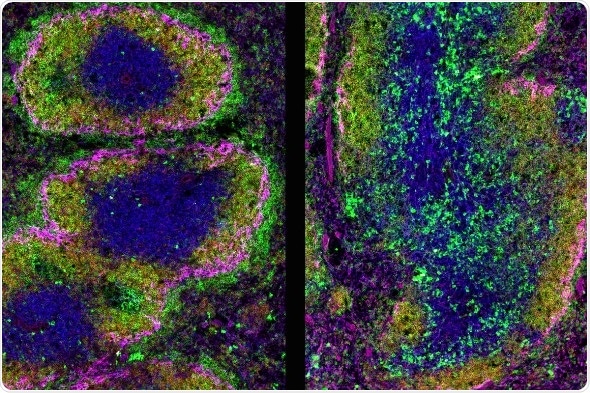
Ikaros loss in B cells causes inflammation in the spleen: Immunofluorescence of spleen sections from 10-week-old mice: Spleen section from mice with Ikaros-sufficient (Ikzf1B+, left panel) and -deficient B cells (Ikzf1B–, right panel). Sections were stained with anti-IgM (green), anti-IgD (red), anit-CD90 (blue), and anti-CD169/MOMA-1 (purple) antibodies. In the presence of the transcription factor Ikaros (left panel), the organized white pulp area is structured into the T cell zone (CD90+ T cells (blue)) and B cell follicles (IgM+IgD+ follicular B cells (green and red)), which are framed by a ring of metallophilic magrophages (anti-CD169+/MOMA-1 (purple)) and marginal zone B cells (IgM+ (green)). Plasma cell (cytoplasmic IgMhi (green)) are sparsely seen in the T/ B cell zone, as well as clustered outside the white pulp area in the red pulp. In spleens with Ikaros-deficient B cells (right panel), T cell zone is expanded and not clearly separated from the B cell zone, while the B cell follicles appear tattered and disorganized. The marginal zone is interrupted and the marginal zone B cells are greatly reduced. The T-cell zone and interfollicular zone is enriched with ectopic IgM staining and bright IgM+ B cells, which could reflect immune complexes and transient plasmablasts, respectively. (Credit: IMP).
B cells are white blood cells that generate antibodies against an almost unlimited number of pathogens, a capacity that is vital for any higher organism. However, establishing a diverse repertoire of pathogen recognition comes at a price, as some B cells will inevitably go wild and turn against the organism’s own healthy tissue.
Such autoreactive B cells need to be silenced and are kept within the pool of B cells for emergencies, such as serious infections, for which no specific B cell can be found in the active B cell pool. Scientists from the lab of the IMP Deputy Director Meinrad Busslinger have investigated the two antagonistic mechanisms that silence or awaken autoreactive B cells. Both mechanisms are controlled by the protein Ikaros, thereby controlling autoimmunity – as now reported in the journal Nature Immunology.
Remarkably, the starting point of the project was not autoimmunity, but an interest in how the transcription factor Ikaros may influence B cell differentiation.
Transcription factors are proteins that bind to specific parts of the DNA to activate or repress certain genes. “When you are interested in how a transcription factor works, you normally start by looking at what it does early or late in B cell development, and you can do so by selectively inactivating the factor”, says Meinrad Busslinger in explaining the chosen approach.
In the absence of Ikaros in mature B cells of mice, the scientists observed a high degree of autoimmunity. This made them turn to the two mechanisms that switch B cells on and off: “Anergy”of the B cell antigen receptor (BCR) is a tolerance mechanism that makes autoreactive B cells insensitive to self-antigens, and “Toll-like receptor (TLR)” signalling awakens B cells.
“We could show that in the absence of Ikaros, BCR anergy decreases, TLR signalling increases, and B cells become hyperactive leading to systemic autoimmunity in the animals”, says Tanja Schwickert, first author of the study, who continued to investigate how Ikaros specifically controls the two mechanisms causing these signalling defects. The results supported the main conclusion of the study: “Ikaros acts as a guardian that prevents autoimmunity.”
The research project started with the development of a mouse model to study the function of Ikaros in mature B cells in 2012.
At this point, nobody could have guessed that we will identify Ikaros as a fundamental regulator of autoimmunity”
Meinrad Busslinger, IMP Deputy Director
He now considers the establishment of this link to be a case of serendipity: “This is how basic research works – you ask a fundamental question, and you work out mechanisms with unexpected, but very far-reaching implications for all sorts of phenomena”. In this case, two mechanisms were discovered through which a single protein is sufficient to control autoimmunity.
In humans, Ikaros mutations have been identified as a risk factor for systemic lupus erythematosus (SLE), an autoimmune disease that cannot be cured and drastically shortens the life expectancy of affected patients. The present study will help to bring this condition into context of a basic mechanism that could be involved in the generation of autoimmune disease.
Source:
Journal reference:
Schwickert, T.A., et al. (2019) Ikaros prevents autoimmunity by controlling anergy and Toll-like receptor signaling in B cells. Nature Immunology. doi.org/10.1038/s41590-019-0490-2.