The majority of the British public understand that fast-tracking vaccines, treatments and tests for COVID-19 could bring greater risks, according to a survey commissioned by law firm Hill Dickinson’s Life Sciences practice.
Two-thirds of respondents agreed that regulators should be able to relax requirements, but more than three-quarters of the 1,000 people surveyed agreed that regulators should balance this with additional long-term monitoring.
The urgent need for new medical interventions to help treat COVID-19 means regulators are adopting temporary measures to accelerate approval processes. The UK’s Medicines and Healthcare products Regulatory Agency (MHRA), for example, has introduced fast-track approval routes for ventilators, PPE and COVID-19-testing kits, and has been expediting clinical trial applications for new treatments for COVID-19.
Whilst there is no reason to believe that accelerated procedures impose lower safety standards, this survey set out to examine UK public perception of risk in the regulatory process. More specifically, it sought to establish whether respondents recognized that changes to regulations designed to protect them, made during an emergency disease outbreak, could have potential safety implications.
Balancing speed to market and patient safety
Nearly two-thirds (64%) of the UK public agree that when a dangerous new disease emerges, as we are currently experiencing with COVID-19, regulators should be able to relax requirements to speed up the development and adoption of new medicines. When questioned further, half of all respondents agreed that new vaccines should be tested on people sooner than usual, even if side-effects are unknown, and 55% agreed that we have to accept that some patients may die due to side effects of COVID-19 treatments not identified due to an accelerated approval process.
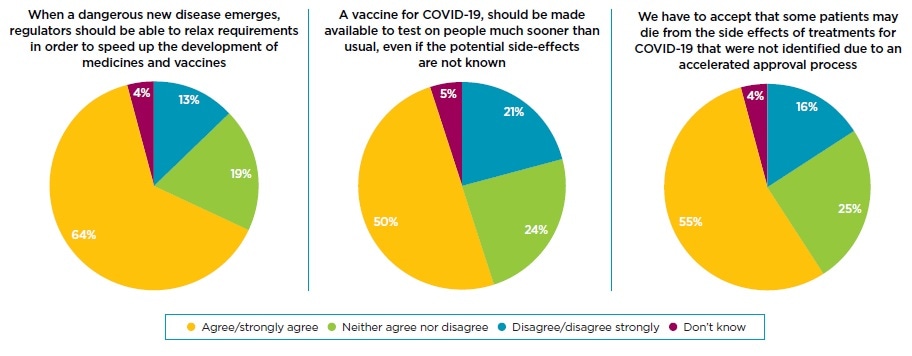
Interestingly, the proportion of respondents that see the increasing health risk as acceptable tends to increase with age. 48% of 18-24 year olds were willing to risk the potential side-effects for more rapid testing, compared with 50% of those aged 65-74 and 63% of those aged 75+. Similarly, 53% of 18-24 years olds were willing to accept that some patients may die from unforeseen side effects, compared with 56% of 65-74 year olds and 75% of those aged 75+.
In comparison to vaccine and medicine development, support for speeding up production of new medical devices (such as respirators) was split, with only 36% agreeing, and the same number disagreeing, that we should relax quality controls to speed up production.
Commenting on the results, James Lawford Davies, Partner at Hill Dickinson, said:
“The UK regulatory process has adapted very quickly in response to the pandemic, enabling rapid scientific advice, review and approval. For example, the MHRA approved the Oxford vaccine trial in just over a week.
“Prioritizing COVID-19 assessments should not compromise the safety of trial participants or the thoroughness of the review, but it is interesting to see how the public perceive the balance of risk and benefit.”
A long-term commitment to monitoring
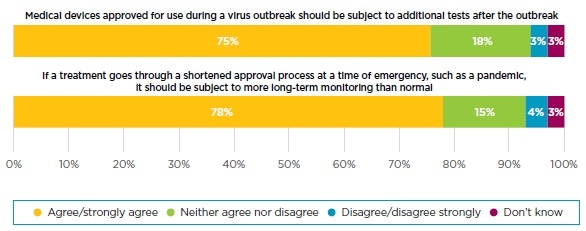
More than three-quarters (79%) of the UK public expect that if a novel treatment goes through a shortened assessment and approval process in a time of emergency, such as a pandemic, it should be subject to more long-term monitoring than usual. Similarly, 75% agree that medical devices approved for use during an outbreak should be subject to additional tests after the outbreak.
There is a perception that an expedited approval process warrants a greater long-term responsibility to monitor approved products, as exemplified by the MHRA’s dedicated Coronavirus Yellow Card reporting system.”
James Lawford Davies, Partner at Hill Dickinson
The Yellow Card reporting system encourages healthcare professionals to report any side effects and adverse incidents that result from healthcare products, medicines or medical devices during COVID treatment using an online form.
Confidence in social distancing and hygiene
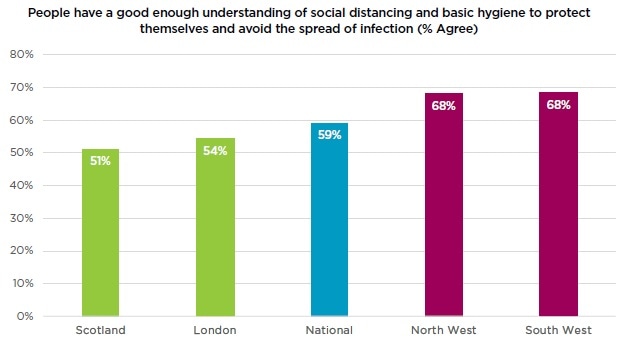
59% agree that people have a good enough understanding of social distancing and basic hygiene to protect themselves and avoid the spread of infection, whilst only 20% disagree. Geographically, respondents in London (54%) and Scotland (51%) were least likely to agree that social distancing and hygiene were understood. By contrast, the South West (68%) and North West (68%) saw the highest levels of agreement.
The fact that a relatively large proportion of the population think people have a good enough understanding of social distancing and hygiene suggests a significant level of confidence in people’s willingness to self-regulate. Time will tell whether this is correct as lockdown is eased.We surveyed the UK public on 20 April which may give some context to the regional variations we observed. However, when we compared levels of agreement by region to the total rates of COVID-19 cases as of 2 June in those regions there was no clear correlation.”
James Lawford Davies
Methodology
Based on a survey of 1022 UK-based adults commissioned by Hill Dickinson and carried out by Instinctif Partners and Dynata in April 2020.