Advances in anti-HIV treatment have thankfully extended the life of people living with HIV (PLWH). However, there are still significant hurdles including co-morbid conditions such as neurocognitive impairment and HIV latency that complicates the lives of PLWH, standing in the way of a cure.
In the early 2000s, with advances in HIV therapy underway, I pivoted from my research in HIV virology/immunology to study the interface between the virus and the brain to address two key questions: 1) what drives neurologic impairment in the brain and 2) what is the role of the brain as a reservoir for HIV.
I took a leap and moved out of my comfort zone as I was not classically trained in neuroscience, however, biomedical training allows me to pose questions of public health concern and tackle them in whichever discipline to get to the answer, which often involves collaborating with experts to gain momentum and learn from.
.jpg)
Image Credit: RAJ CREATIONZS/Shutterstock.com
How does HIV attack the immune system and why is it so difficult to treat?
This question can be answered in relation to how HIV attacks the immune system under no treatment vs after treatment. Without controlling HIV load, the virus predominantly infects CD4+ T cells causing their depletion, as well as inducing hyper inflammation leading to CD8 cytotoxic T cells becoming either senescent or ineffective in killing HIV infected cells.
HIV can essentially perturb most cells in the body either directly or indirectly. Under potent viral control, immune activation still persists and the question is what is driving this persistent immune activation? Is it a residual virus in lymph nodes/other organs where anti-HIV drug penetration is less ideal?
Low-level HIV treatment can be a driving factor as well as HIV hiding in organs that are not as easily accessible to treatment such as the brain.
What do your findings show about astrocytes and their role in HIV infection?
Infection of astrocytes has been controversial and most of these studies either relied on a single round of replication assays that do not assess HIV integration into these cells or used full virus evaluated under in vitro systems.
Humanized mice that support HIV infection still have mouse origin of astrocytes that are not infected by HIV. Here, we did two things:1) developed a humanized mouse model that has human astrocytes and 2) used postmortem brain samples from HIV+ donors to probe for the extent of HIV infection of astrocytes.
In both systems, we showed that HIV infects astrocytes, albeit to a lower extent at 1-3% of astrocytes than more susceptible cells for HIV.
The sheer number of astrocytes, in the billions of cells in a human brain, indicates that they can contribute significantly to the HIV reservoir, and most importantly the virus in these cells can be trafficked back to the periphery through infiltrating T cells.
In essence, astrocytes may have bursts of HIV that can mediate dysregulation in the brain and also be circulated back into peripheral organs through the trafficking of immune cells in and out of the brain. This is significant as it may explain viral blips among HIV infected patients and/or persistent low levels of HIV in the brain that is a hurdle to a cure.
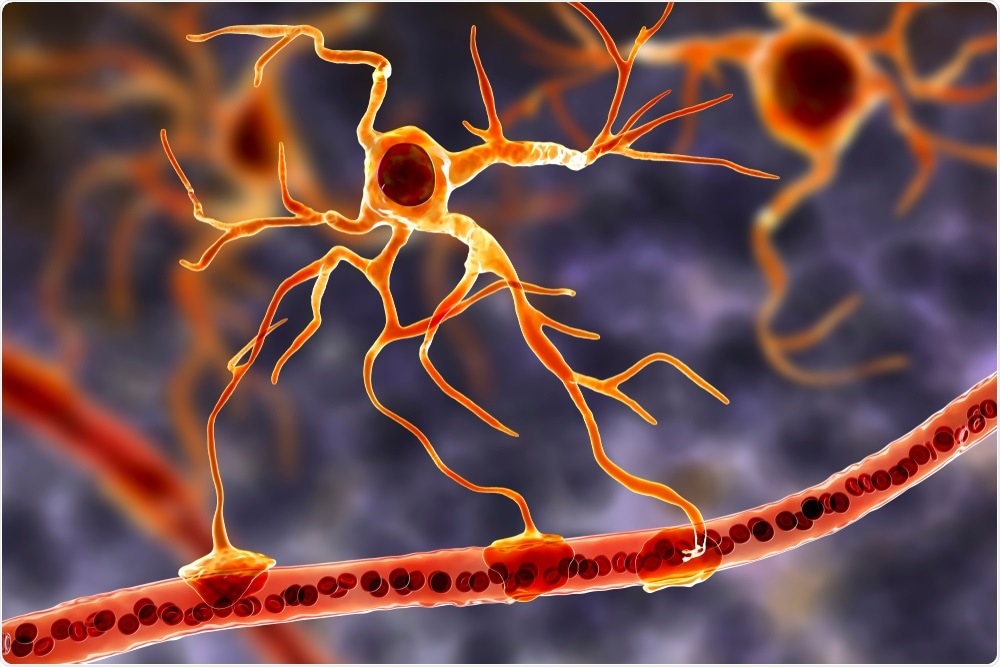
Image Credit: Kateryna Kon/Shutterstock.com
Why does it become dangerous when the HIV virus migrates to peripheral organs?
I would not necessarily use the word dangerous, rather the study shows that HIV in the brain cannot be viewed as a virus that is isolated in this organ and does not interact with other organs.
These burst of virus that comes from the brain can reseed peripheral organs, playing a role in persistent inflammation and/or viral blips (spikes of the virus under cART).
What is cART and what are its limitations?
cART is a combination of antiretroviral drugs that have transformed HIV from a death sentence to chronic disease. Typically, it consists of three drugs that interfere with various stages of the HIV life cycle, and together they can achieve maximum suppression of HIV, but not a cure. If cART is interrupted, the virus rebounds fairly quickly.
cART can have toxic effects and some regiments may be more “brain-friendly” than others. For example, efavirenz (EFV) is linked to neurotoxicity and even psychotic episodes.
cART drug concentrations tend to also be lower in the CNS due to the blood-brain barrier, and this is also observed in other organs as well. Therefore, lower drug concentrations may allow for residual virus replication that is probably below the limit of detection of our current assays.

Image Credit: Maxim Ermolenko/Shutterstock.com
What do your findings suggest about the mechanism of cure strategies?
The cure strategies are heavily focused on the role of resting CD4+ T cells, which are important but do not represent the whole story of HIV reservoirs and latently infected cells. This focus has undervalued the role of other cells such as brain cells when probing for HIV reservoir and sanctuary sites for HIV.
As a cautionary note, the cure strategies under investigation do not take into consideration the potential determinantal effects of the strategy on highly sensitive organs such as the brain. For example, the shock and kill strategy (to reactive the virus and potently treat the emerging virus) or block and lock (trap the virus in a true latent state).
These approaches in terms of how these agents would even enter the brain are not being discussed, let alone how reactivating the virus in the brain (even if it is transient), or keeping the virus locked, can significantly impact the health of resident brain cells, especially neurons and astrocytes.
How could this research be used to develop better HIV cure strategies?
I do not believe that we will ever have an effective cure strategy that does not address all sanctuary sites for HIV. The brain is especially challenging to study because it is hard to probe.
So, realizing that the brain plays a role as a sanctuary site for HIV places a greater emphasis on the collective scientific community to begin to ask the question of 1) can this specific strategy penetrate the brain? And 2) if it does, what negative effect might it have. We do not have the answers but at least we need to start by asking the questions.
How far do you think we are from an effective cure and vaccine for HIV?
Regrettably, I am not highly optimistic about an effective cure strategy and even a vaccine in the short term. This virus integrates into the host genome, making it very difficult to purge. So even a single copy over time can be problematic.
I think given the cost of cART, concerns over none compliance, and potential toxic effects, we have to keep pushing to put the best science forward for a cure and vaccine.
.jpg)
Image Credit: PhotobyTawat/Shutterstock.com
Will this research influence understanding of viruses and their mechanisms in general?
I hope so if nothing else I hope that it will convince the greater HIV research community that the brain cannot be neglected as we try to understand HIV pathogenesis and continue to devise strategies for a cure.
Could discoveries about HIV benefit our knowledge of COVID-19 and vice versa?
Absolutely, I have a manuscript under review titled “What HIV in the brain can teach us about COVID-19 neurologic complications”.
There is growing evidence to demonstrate that SARS-CoV-2 causes neurologic impairments and it is found in the brain.
Studying HIV, how it enters the brain, mechanisms it perturbs to lead to CNS dysfunction can inform future studies on how SARS-CoV-2 causes these CNS abnormalities
.jpg)
Image Credit: narci5/Shutterstock.com
Where can readers find more information?
Correction to: Astrocytes as an HIV CNS reservoir: highlights and reflections of an NIMH-sponsored symposium. Al-Harthi L, Joseph J, Nath A.J Neurovirol. 2019 Aug;25(4):616. doi: 10.1007/s13365-019-00726-1.PMID: 31041681
About Dr. Lena Al-Harthi
Dr. Lena Al-Harthi is a Professor and Chair of the Dept. of Microbial Pathogens and Immunity at Rush University Medical Center in Chicago, IL.
Her research is focused on HIV/host interactions, with a special emphasis on bridging basic and clinical science in the HIV/AIDS field. Through her multidisciplinary training in HIV-related virology, immunology, and for the past ten years in neuroHIV, she has been able to probe mechanistic questions that are clinically relevant to HIV/AIDS.
Her primary research focus is to understand mechanisms driving HIV-Associated Neurocognitive Disorders (HAND) and the contribution of the CNS to the HIV reservoir. Specifically, she studies the role of astrocytes as a reservoir for HIV and the dynamic cross-talk between the Wnt/b-catenin signaling, inflammatory mediators, and HIV as they converge to impact HIV neuropathogenesis and latency.
She demonstrated that HIV replication in astrocytes is restricted at the level of transcription, largely due to robust expression of Wnt/b-catenin signaling in astrocytes which disrupts HIV LTR activity.
In response to inflammatory signals and even HIV proteins, Wnt/b-catenin signaling is reduced in astrocyte allowing for enhanced HIV transcriptional activity, albeit the level of infection is still low to approximately 5%. However, given the total number of astrocytes in the brain, this percentage can have a great impact on HIV reservoirs.
Further, she demonstrated that the HIV LTR in astrocytes demonstrates properties of HIV latency including restriction at the epigenetic level. She has over 80 peer-reviewed publications, invited reviews/book chapters, and had the privilege to serve on numerous federal and non-federal study sections, including chairing the neuroAIDS and End-Diseases (NAED) study section.
She is also actively engaged in training graduate students, post-docs, and junior faculty and is currently the director of an NIH funded training grant for Ph.D. underrepresented minority students at Rush University.