The COVID-19 pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is known to affect the elderly and those with coexisting chronic diseases such as obesity and diabetes more severely.
With infections continuing to rise in many countries and the potential for continuing viral persistence in the absence of a vaccine, there is an urgent need to understand SARS-CoV-2-mediated pathology better. Key to this are efforts examining human tissues potentially susceptible to infection.
Now, a new study published on the preprint server bioRxiv* reports that even though the fatality rate is significantly higher in diabetics, there is no evidence that this is due to direct viral infection of pancreatic beta cells, which produce the key hormone in glucose homeostasis, namely insulin.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Systemic Viral Dissemination and Disease
Many studies have focused on the lung and cardiovascular implications of the disease, but reports of liver, spleen, kidney, intestine, and brain involvement are piling up. One burning question has been whether the virus can infect the pancreas, causing or worsening glycemic control in type 1 or type 2 diabetes. Prior research has shown that amylase and lipase levels are high in blood samples taken from COVID-19 patients, while glucose control is impaired. Indeed, hospitalized patients develop diabetic ketoacidosis very commonly, indicating the shift in blood sugar levels to the higher side.
Earlier studies also show that diabetic patients are at increased risk of death with COVID-19, new clusters of type 1 diabetes are showing up in some localities, in temporal relationship to COVID-19, and pancreatic cells appear to express the host receptor, angiotensin-converting enzyme-2 (ACE2), potentially allowing the virus to infect and destroy them.
ACE2 Expression in the Endocrine Pancreas
Various earlier studies have reported contrasting results for ACE2 expression in the pancreatic tissue. Very few autopsy studies of pancreatic tissue have been performed since this tissue rapidly breaks itself down, as well as a marked lack of interest in this tissue. The current study thus aimed to use multiple ACE2 antibodies and to use a larger number of donor samples to mitigate some of this confusion.
The researchers from the University of Florida, Indiana University, Icahn School of Medicine at Mount Sinai, Louisiana State University, Baylor College of Medicine and the University of Miami focused on the effect of SARS-CoV-2 infection on β-cells on type 1/type 2 diabetes mellitus (T1D or T2D). They examined ACE2 expression in both exocrine and endocrine cells of the pancreas, but particularly the islet of Langerhans, which houses the β-cells.
Using publicly available single-cell RNA sequencing (scRNAseq) data, along with fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and immunofluorescence (IF) studies to directly observe gene and protein expression of ACE2, they looked for this protein in human tissue using four commercially available ACE2 antibodies with IHC/immunoblot controls. They also evaluated the expression of the viral nucleocapsid (N) protein in pancreatic tissues from deceased COVID-19 patients derived from post-mortem studies.
Temporal Pattern of ACE2 Expression
The researchers also examined tissue from 36 COVID-19-negative donors who did not have diabetes, aged 0-72 years. They found that the ACE2 staining percentage of tissue increases steadily from birth to a peak in the adolescent years, to plateau in early adult life and finally decline over the age of 50 years.
At all ages, ACE2 is expressed in pancreatic duct epithelial cells and microvasculature within the endocrine regions, but not on α-cells or β-cells. These findings were confirmed using scRNAseq and smFISH gene expression data.
Low Levels of ACE2 in β-Cells
The researchers looked simultaneously at scRNAseq data from five datasets, including 22 non-diabetic and 8 type 2 diabetics, finding low ACE2 expression in most islet cell subsets. This showed that normal pancreas from donors without COVID-19 expresses ACE2 mainly in duct cells and endothelium of the small blood vessels.
For the donor samples, in patients without diabetes, less than 2% of cells in the pancreas expressed ACE2, at 4% of acinar cells and over 5% of ductal cells. Type 2 diabetics showed ACE2 expression in 8% of both acinar and ductal cells. ACE2 expression in the islet cells was thus comparably low between diabetic and non-diabetic donors, while TMPRSS2 expression was above 50% in acinar and ductal cells. However, the expression of TMPRSS2 was low in most endocrine cells in the pancreas.
Thus, they found that both ACE2 and the cell protease TMPRSS2 were expressed at low levels in human pancreatic cells and the islet’s β-cells. These findings were confirmed using direct visualization methods.
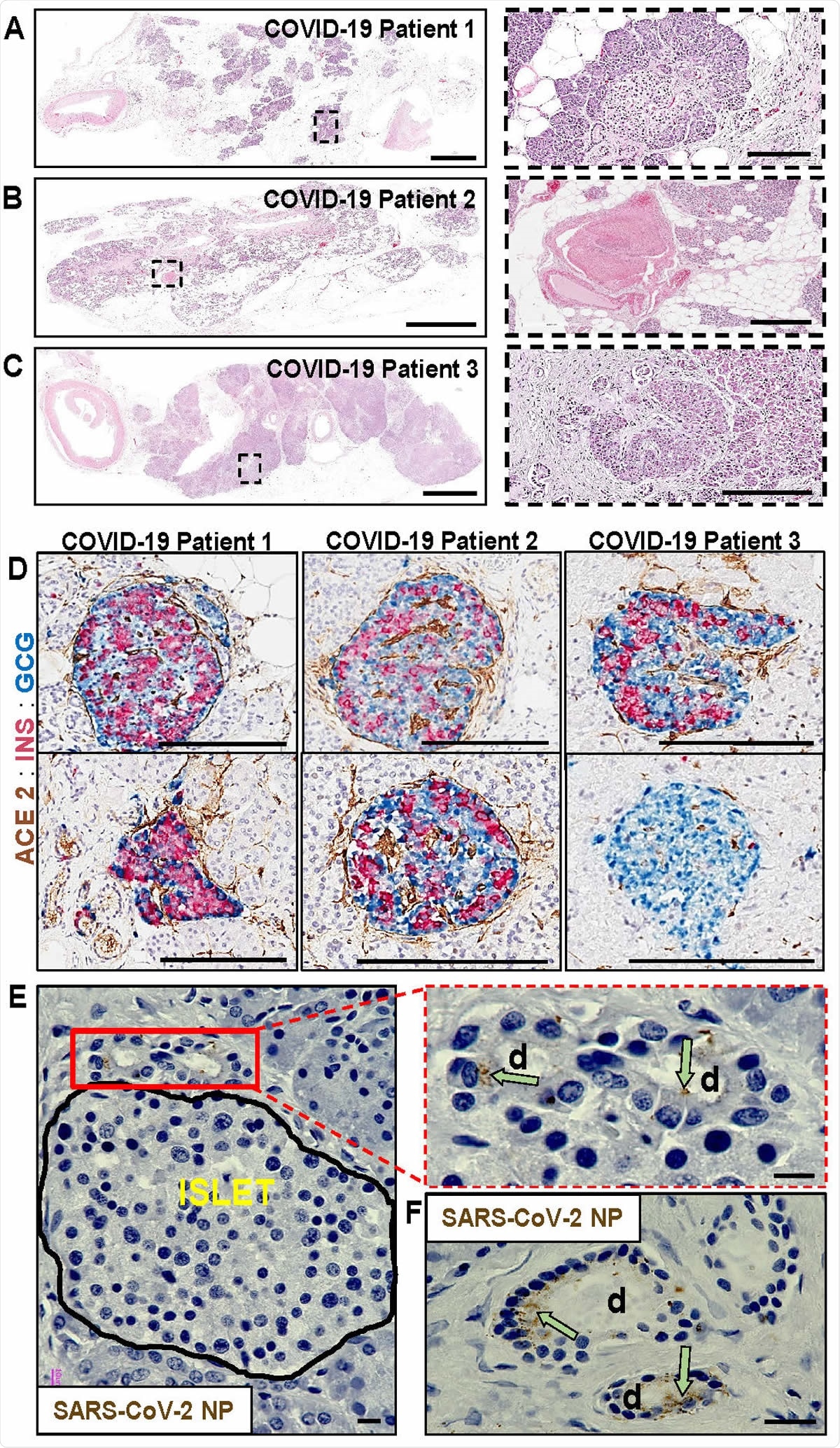
Pathological changes in pancreata of COVID-19 patients. (A) Pancreas tissue section from COVID-19 Patient 1 stained for H&E. Inset highlights fibrotic center with residual acinar cells and islet surrounding ductules. Scale bars: 3mm, inset 200µm. (B) Pancreas tissue section from COVID-19 Patient 2 stained for H&E. Inset highlights microthrombus without adjacent hemorrhages. Scale bars: 4mm, inset 400µm. (C) Pancreas tissue section of COVID-19 Patient 3 stained for H&E. Inset highlights a large, irregularly shaped pancreatic islet surrounded by fibrotic tissue. Scale bars: 4 mm, inset 200µm. (D) Representative pancreas tissue sections from three COVID-19 patients stained for ACE2, insulin (INS) and glucagon (GCG). Scale bars: 200µm. (E) SARS-CoV-2 NP observed in intralobular ducts (d) near an islet in the pancreas of COVID19 Patient 1. Scale bars: 10µm. (F) Representative image of multiple ducts showing SARS-CoV-2 NP positivity in the pancreas of COVID-19 Patient 1. Scale bar: 20µm.
Studies of Autopsy Tissue From COVID-19 Patients.
The researchers also looked at pancreatic tissue retrieved by autopsy of three patients with fatal COVID-19, aged 45-72 years. Two of these patients were known to have type 2 diabetes. They found that in the non-diabetic patient, fatty replacement of the acinar cells, with islets within the fibrotic regions. The other patients showed moderate to numerous islets.
They comment, “These histopathological findings were compatible with the normal range of expected lesions within the exocrine compartment in pancreata from aged patients and those with T2D.”
Direct visualization by IHC showed moderate ACE2 expression in duct epithelium and no evidence of viral N protein in the endocrine pancreatic tissue. Instead, N protein was localized to the duct epithelium, widely scattered through the exocrine pancreas, and there were several thrombotic lesions.
Implications
This information seems to rule out direct viral infection of endocrine β-cells of the pancreas as the reason for the clusters of new diabetes cases or the increased mortality in people with diabetes with COVID-19. Indeed, the duct epithelium and microvascular endothelium seem to be more likely viral targets.
Another paper contradicts these results, demonstrating not only that ACE2 is expressed in endocrine cells within human pancreatic islets isolated from the pancreas. The small size of the current study may not allow susceptibility of the pancreas to be ruled out or confirmed. One possibility is that islet isolation is a process that may affect the expression of ACE2, or that exposure to higher doses of the virus may facilitate islet cell infection in an ex vivo setting.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources