SARS-CoV-2 is the agent that causes coronavirus disease 2019 (COVID-19), and Mpro is an essential protein required for the life cycle of coronaviruses.
Currently, there is an intense interest in discovering effective inhibitors of SARS-CoV-2 Mpro. Still, it is not clear whether the versions known so far would be safe and effective in treating human coronavirus diseases.
For example, one currently available compound contains an aldehyde reactive group (warhead), but aldehydes are generally considered toxic due to off-target reactions.
Mpro inhibitors that use ketoamide or “Michael acceptor” warheads instead are considered preferable in terms of specificity and stability, but they appear insufficiently effective to be of therapeutic benefit.
Now, Michael Lin (Stanford University) and colleagues have developed a ketoamide inhibitor, called ML100, that suppresses SARS-CoV-2 replication more effectively than previously described ketoamide or Michael acceptor compounds.
Furthermore, ML1000 was designed using chemical motifs from drugs with well-established safety profiles in humans.
“Our findings identify ML1000 as a promising new pre-organized scaffold for the development of anti-coronavirus drugs,” writes the team.
A pre-print version of the article is available ion the server bioRxiv*, while the article undergoes peer review.
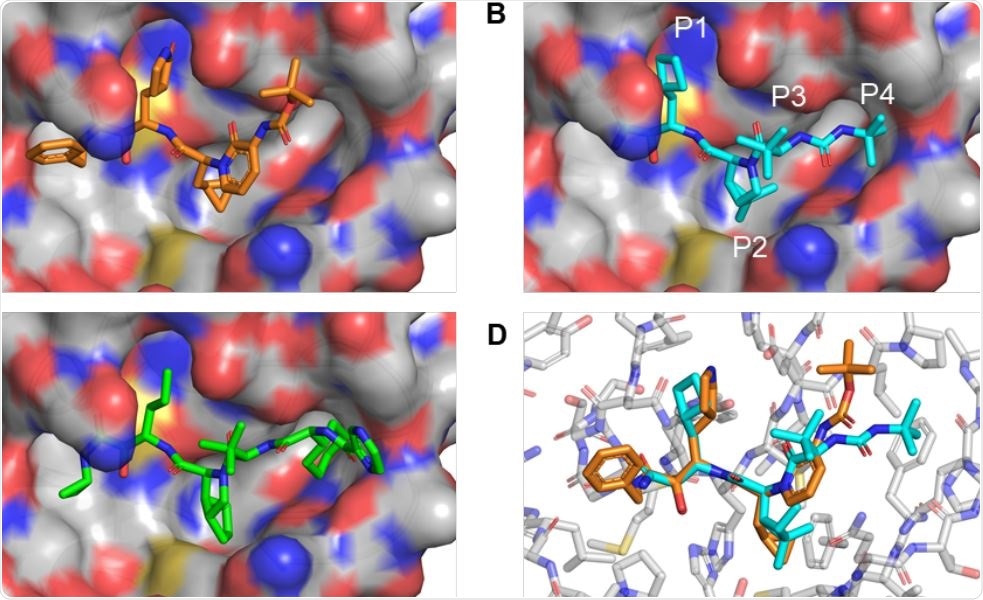
HCV protease inhibitors with a P2 proline analog can be docked into the SARSCoV2 Mpro active site. (A) Co-crystal structure of SARSCoV2 Mpro and inhibitor 13b (PDB 6Y2G). (B) Using Pymol, boceprevir was placed into the SARSCoV2 Mpro active site and unconstrained bonds were manually rotated for optimal complementary with the S1 and S2 pockets and hydrogen-bonding to the backbone carbonyl of Glu-166. (C) Telaprevir was similarly docked into the SARSCoV2 Mpro active site for optimal complementary with the S1, S2, and S4 pockets and hydrogen-bonding to the backbone carbonyl of Glu-166. (D) Alignment of the 13b-Mpro cocrystal with the manually docked boceprevir structure shows that the backbone of the P2-analogous segment of 13b is superimposable with the proline analog of boceprevir.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Antivirals urgently needed in the absence of a vaccine
In the absence of an effective vaccine against SARS-CoV-2, antiviral drugs will be needed to reduce severe disease and death, as the current COVID-19 pandemic continues to sweep the globe.
Ideally, these drugs could be administered orally so they can be taken in the outpatient setting, without people needing to be hospitalized.
However, the only antiviral to date with proven efficacy – remdesivir – must be administered intravenously in hospital.
“Thus, drugs that can effectively suppress coronavirus replication in an outpatient setting during the entire post-infection period do not exist, but are urgently needed,” says the researchers.
Mpro inhibitors have become an important focus
Inhibitors of Mpro, a key protein needed for the coronavirus life cycle, have become an important focus in the search for effective antiviral agents.
However, whether coronavirus Mpro inhibitors have the necessary properties to be safe and effective against human coronaviruses remains unclear.
One compound, called GC-376, for example, has an aldehyde warhead, but aldehydes are generally not considered ideal due to off-target reactions that result in toxicity.
Mpro inhibitors with ketoamide or Michael acceptor warheads, on the other hand, exhibit better specificity and stability than aldehyde warheads, but they do not appear to be effective enough to provide therapeutic benefit.
For example, one compound called 13b, which has a ketoamide warhead, demonstrates a 50% inhibitory concentration (IC50) on Mpro in vitro of 670 nM, but the 50% effective concentration (EC50) in blocking SARS-CoV-2 replication in human cells is only around 4 μM.
This compares unfavorably to a concentration of less than 0.1 μM that was achieved in mouse lungs, say Lin and colleagues.
Another compound called N3, which has a Michael acceptor warhead, demonstrates a low IC50 in vitro, but its EC50 for SARS-CoV-2 replication is 17 μM, suggesting poor cell permeability.
“Thus, the development of new Mpro inhibitor designs with low toxicity and low EC50 in cells remains an urgent priority,” writes the team.
Incorporating cyclic structures to improve affinity
The researchers say that many clinically approved protease inhibitors incorporate cyclic structures to improve affinity by pre-organizing the inhibitors into a conformation that is favorable for binding.
In the current study, while visualizing the co-crystal structure of 13b and SARS-CoV-2 Mpro, the researchers noticed that 13b adopts a pronounced kink in its main chain at the P2 residue.
Given previous work the researchers had carried out with small-molecule inhibitors of the hepatitis C virus, they recognized that this kink resembled the one formed by proline analog rings in the clinically approved anti-HCV drug boceprevir.
On performing initial tests, the researchers found that boceprevir did indeed exhibit activity against Mpro.
Next, Lin and the team explored the ability of a cyclic moiety to improve the affinity of coronavirus Mpro inhibitors.
Assuming that a pre-organized backbone conformation could be beneficial, the team designed new ketoamide-based Mpro inhibitors based on central proline rings.
ML1000 suppressed SARS-CoV-2 replication at sub-micromolar concentrations
One of the new inhibitors, ML1000, with a molecular weight of 549 Da and an IC50 value of 12 nM on SARS-CoV-2 Mpro inhibited viral replication in human cells with an EC50 of 0.1 µM, thereby representing the highest-potency non-aldehyde Mpro inhibitor reported to date.
“ML1000 can be considered an example of a new class of coronavirus Mpro inhibitors that feature a cyclic P2 group, high affinity, and solubility,” say Lin and colleagues.
“ML1000 represents a new structural class of potent ketoamide inhibitors of coronavirus Mpro, and may serve as a promising starting point for the development of drug candidates against human coronavirus diseases,” the team concludes.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Lin M, et al. Rational design of a new class of protease inhibitors for the potential treatment of coronavirus diseases. bioRxiv, 2020. doi: https://doi.org/10.1101/2020.09.15.275891