The viral transmission underlying the current COVID-19 pandemic has been a matter of intense investigation. According to the World Health Organization (WHO), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a respiratory virus, and transmission is by respiratory droplets and aerosols from infected individuals. A new study published on the preprint server medRxiv* in October 2020 shows that the virus also multiplies robustly in the oral cavity, indicating that saliva plays a role in viral transmission.
It is now accepted that breathing, singing, coughing, and sneezing are linked to SARS-CoV-2l transmission. All of these involve air passing through the mouth, but most research has explored infectious particles' movement from the lungs and the nose to the outside to possibly infect others.
Among 40 studies, including over 10,000 COVID-19 patients, however, there was a range of oral manifestations, such as ageusia, dryness of mouth, and blisters on the mucosal lining, in half of all cases.
Saliva contains viral RNA, and as a result, is being employed as a diagnostic test sample. However, if the oral cavity and associated salivary glands transmit the virus through saliva to the lung and the gut, saliva could also be of importance in carrying the virus to others.
Susceptibility of Oral Tissues
Therefore, the current study sought to examine the infection and replication of the virus in oral tissues, including the mucous membrane and the salivary glands. Viral entry depends on host receptors like ACE2 and TMPRSS2, which have poorly described but variable expression patterns in oral tissues. The oral cavity contains a wide variety of cells adapted to feeding, digestion, and speech.
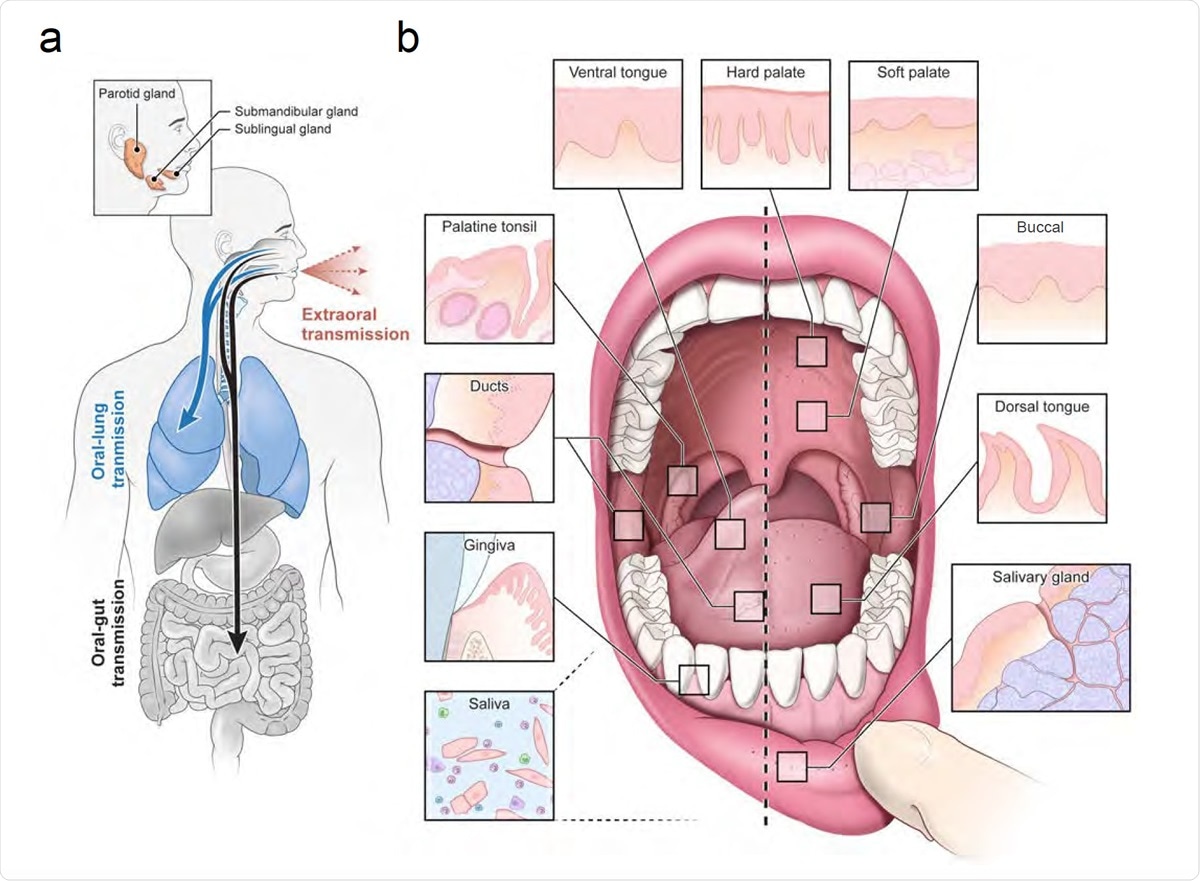
An infection and transmission axis for SARS-CoV-2 among distinct oral niches. (a) The contribution of the oral cavity to COVID-19 pathogenesis and transmission has been little explored. It is unknown whether SARS-CoV-2 can infect and replicate in the oral mucosa or glands. This is critical because if the glands or mucosa are sites of early infection, they may play an important and underappreciated role in transmitting virus “intermucosally” to the lungs or gastrointestinal tract. Alternatively, saliva may also play a central role in transmitting the virus extraorally in asymptomatic, pre-symptomatic, or symptomatic individuals. (b) The human oral cavity is a diverse collection of tissue niches with potentially unique vulnerabilities to viral infection. These sites include oral mucosae (hard palate, buccal mucosa, dorsal and ventral tongue) as well as the also the terminally differentiated secretory epithelia of the minor saliva glands (distributed in the buccal and labial mucosa, hard and soft palate, ventral and dorsal tongue) and major saliva glands (parotid, submandibular, and sublingual). Nearby are diverse oropharyngeal niches (palatine and lingual tonsils, soft palate). Saliva, a mixture of fluids, electrolytes, proteins, and cells (immune and sloughed mucosal epithelial cells) is made primarily by the saliva glands and empties into the oral cavity where it mixes with other fluids (crevicular fluid) and cells.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Heterogeneous Cell Types
The researchers first generated a human oral single-cell RNA sequencing atlas, the first-ever, to help predict which cell types might promote SARS-CoV-2 infection. They found that in just the minor salivary glands (SGs) and the gums, there were 50 cell types.
The former had epithelial types belonging to the serous and mucous acini, salivary ducts, myoepithelial cells, and glia, besides ionocytes, fibroblasts, endothelial, and smooth muscle cells. There were also types of immune cells, including plasma cells and B cells, T cells, and macrophages. The gums also showed dendritic and other immune cell types and various types of epithelium.
Viral Entry Receptor Expression
They then used in situ hybridization (ISH) to examine the pattern of ACE2 and TMPRSS2 expression in various epithelial cell types in the SGs and mucosal cells. Based on this, they predicted that SARS-CoV-2 infection could occur in multiple epithelial types, especially the SG ducts and acini, and the uppermost layers of the mucosal epithelium.
This was confirmed on autopsy and outpatient samples of the oral and oropharyngeal mucosa, using ISH and confirmatory polymerase chain reaction (PCR) testing for viral RNA. This proved that the mucosa are sites of SARS-CoV-2 infection, with SGs being susceptible to infection. The shed epithelium could provide potential routes for the virus to spread to other parts of the body through the saliva.
Two SG responses are possible, the first being a response to SARS-CoV-2 infection by shedding the infected cells and reducing gene activity involved in viral protein transcription. The other may allow the virus to replicate in a sheltered environment, leading to persistent and symptomatic infection.
Oral Infection, Shedding in Saliva, and Symptoms
The researchers also carried out a prospective study in an outpatient cohort, using nasopharyngeal mucosa and saliva samples. They looked for correlations between the viral burden in saliva, viral RNA in the shed oral epithelial cells, and the presence of symptoms suspicious of COVID-19.
They found that some patients took more than two months to clear the virus from saliva and nasopharyngeal samples. Asymptomatic subjects also carry the virus for long periods of time. In some cases, the nasopharyngeal samples were negative for the virus, while saliva continued to be positive, indicating sustained viral shedding from either infected SGs or infected epithelial cells.
Finally, the detection of salivary SARS-CoV-2 RNA predicted anosmia and ageusia associated with a high viral load and epithelial cell infection. The use of masks decreased expelled salivary droplets by a factor of over 10. Further study will show if this is equivalent to reduced viral RNA as well.
Implications
Overall, this study demonstrates the susceptibility of the oral mucosa and SGs to this virus and the presence of actual infection. Transmission of the virus via saliva will require public health measures to block salivary dispersion.
Moreover, the integrated oral human atlas showed the broad susceptibility of minor SGs to SARS-CoV-2. These glands are spread over the tongue, palate, and mucosa, all of which are "hotspots for SARS-CoV-2 infection." This correlates well with and may help explain why COVID-19 patients often lose their sense of taste and complain of a dry mouth.
The occurrence of asymptomatic but productive infection at most of these sites could also explain why silent spread occurs so frequently in COVID-19. Again, comparison with mouse oral cavity single-cell data showed that the expression of both ACE2 and TMPRSS2 increased as cells in the epithelium moved from the bottom towards the top, poised to eventually shed into the saliva. This would favor and explain asymptomatic COVID-19 since shed cells do not produce any specific symptoms.
On the other hand, these infected epithelial cells can induce a strong local immune response in both the gut and the mucosa, with antibodies being secreted by the oral mucosa and the SGs. This corresponds to the observed link between the presence of oral infection and a robust antibody response in saliva.
The authors conclude: “The discovery of an oral source of infection and replication in the SG as well as the natural conduit for viral spread via saliva establishes the possibility of two infection axes in COVID-19.”
Future Directions
The study raises the need for testing both nasopharyngeal and oral samples to assess the spread of SARS-CoV-2. Secondly, the authors point out the possibility that the virus spreads not from the nose to the mouth through respiratory mucus but from the mouth, which is infected via fomites or droplets. Thirdly, the above question also leads to further confusion as to whether the route of primary infection affects the clinical severity and the host immune response.
Daily testing using both nasopharyngeal swabs and salivary testing will be needed to help answer these questions. This study's importance is providing a proper understanding of asymptomatic spread, which has been the bane of all containment efforts, is obvious. The findings also support the relevance of universal hand hygiene, face mask use, and social distancing to prevent transmission via salivary droplets and aerosols and fomites contaminated by saliva.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Byrd, K. M. et al. (2020). Integrated Single-Cell Atlases Reveal an Oral SARS-CoV-2 Infection and Transmission Axis. medRxiv preprint. doi: https://doi.org/10.1101/2020.10.26.20219089. https://www.medrxiv.org/content/10.1101/2020.10.26.20219089v1
- Peer reviewed and published scientific report.
Huang, Ni, Paola Pérez, Takafumi Kato, Yu Mikami, Kenichi Okuda, Rodney C. Gilmore, Cecilia Domínguez Conde, et al. 2021. “SARS-CoV-2 Infection of the Oral Cavity and Saliva.” Nature Medicine, March, 1–12. https://doi.org/10.1038/s41591-021-01296-8. https://www.nature.com/articles/s41591-021-01296-8.