Mitigation strategies have been implemented to control the COVID-19 (coronavirus pandemic) pandemic. Since late December 2019, the causative agent, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 111 million individuals and claimed more than 2.47 lives. One year on, the first individuals are being administered vaccines against the virus.
Several types of vaccines are under investigation to address all challenges, such as production capacity, logistics, cost, immune variant escapes, etc. Therapeutic options are also highly limited for the heterogeneous COVID-19.
In the search for a broadly acting pan-specific antiviral, an interdisciplinary team from Germany tested the previously proposed (for antiviral treatment against Influenza A infections) - Influenza A virus (IAV) defective interfering particles (DIPs). The research is published online on the bioRxiv* preprint server.
Due o a large deletion in their genome, the IAV DIPs cannot replicate. Also, the DIPs suppress and interfere specifically with homologous viral replication in a co-infection scenario, known as replication interference. Because of this replication interference, the IAV DIPs protect mice against an otherwise lethal IAV infection. The IAV DIPs also stimulate innate immunity rendering protection against viruses such as the influenza B virus and the pneumonia virus of mice (PVM).
Based on these observations, the researchers proposed the IAV DIPs as an effective antiviral agent for the treatment of COVID-19. They tested its efficacy against SARS-CoV-2 in vitro and found the (JAK/STAT) signaling involved.
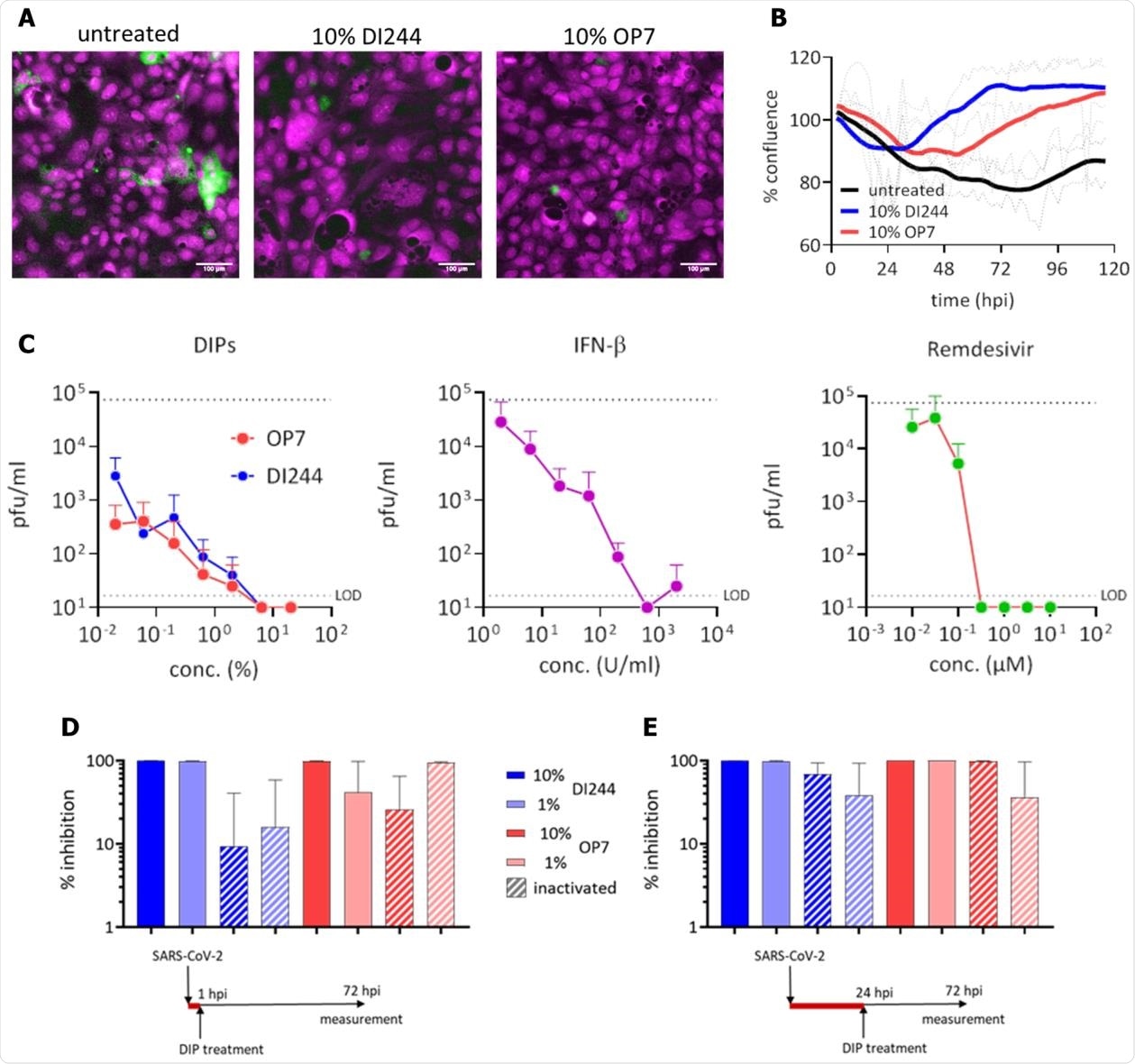
Inhibition of SARS-CoV-2 replication and spreading by IAV DIPs. SARS-CoV-2-infected Calu-3 cells (MOI=0.03) were treated with IAV DIPs (DI244 or OP7), IFN-β, or remdesivir at 1 hour post infection (hpi). For DI244 and OP7 treatment, highly concentrated produced, cell culture-derived DIP material (Hein et al., 2021)(Hein et al., submitted) was used. % (v/v) indicates the fraction with respect to the cell culture volume of 100 µL. Stock concentration, 5.6 x 108 and 1.12 x 1011 167 DI vRNAs/mL for DI244 and OP7, respectively. (A) Immunofluorescence analysis of the SARS-CoV-2 S protein expression (green, magenta: DNA) at 3 dpi. Scale bar, 100 µm. (B) Cytopathic effect. Confluence (% of initial) was measured by live-cell microscopy at 2 h intervals. Thick lines represent smoothened data (Savitzky-Golay filter), dotted lines show SD of original data (n=2, independent experiments). (C) Effective concentration range of DI244 and OP7 compared to IFN-β and remdesivir. Viral titers were determined from the supernatant at 3 dpi by plaque assay. Upper dotted line indicates virus titer in untreated cells, lower dotted line shows the limit of detection (LOD). Independent experiments were conducted; mean +/- SD (n=3) is shown. (D) and (E) SARS-CoV-2 growth inhibition by inactivated DIPs. SARS-CoV-2 infected cells were treated with active or UV inactivated DIPs at 1 hpi (D) or 24 hpi (E). Percentage inhibition of viral growth relative to mock treatment is shown; mean +/- SEM (n=3) is depicted.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The JAK/STAT is a signaling pathway involved in the immune response, cell division, cell death, and tumor formation. The innate immunity is induced via the Janus kinase/signal transducers and activators of transcription (JAK/STAT).
While it is reported that the SARS-CoV-2 replication modulates and inhibits the interferon (IFN) response, the addition of the IFN inhibits the SARS-CoV-2 replication. However, the use of IFN for treatment is cost-intensive and also poses the risk of side-effects.
The researchers wondered whether the IAV DIPs would be able to suppress the SARS-CoV-2 replication through their ability to stimulate a physiological IFN response in the infected target cells.
To test this, they produced two promising candidate DIPs: a prototypic, well-characterized conventional IAV DIP “DI244” and a novel type of IAV DIP “OP7” containing point mutations instead of a large internal deletion in the genome using a cell culture-based production.
For the in vitro studies, they used Calu-3 cells (human lung cancer cell-line) to co-infect with SARS-CoV-2 and DI244 or OP7, respectively. Both the DIPs completely inhibited the SARS-CoV-2 replication and spread in a range comparable to IFN-β or remdesivir treatment.
The researchers found that the SARS-CoV-2 replication is abrogated by IAV DIP treatment in vitro. They also established that the stimulation of innate immunity causes this inhibition.
While a range of treatment options (the polymerase inhibitor remdesivir, monoclonal antibodies, or an antibody cocktail, corticosteroid dexamethasone, etc.) have been tried during this pandemic, the treatment of COVID-19 patients with the IFNs has not been approved yet.
It is known that the SARS-CoV-2 infection modulates and inhibits the IFN response. All IFNs (type I, II, and III) exhibited potent antiviral activity with SARS-CoV-2 replication in vitro.
In this study, the researchers observed that the UV-irradiated and thus inactive DIP material showed a residual inhibitory effect, indicating a specific activity of IAV DIPs in the SARS-CoV-2 suppression
The researchers stated that the IAV DIps confer protection in general against other heterologous IFN-sensitive respiratory viruses. The IAV DIPs as an effective antiviral agent can potentially suppress the replication of new variants of SARS-CoV-2.
“Considering the emergence of new SARS-CoV-2 variants that render the efficacy of various vaccine candidates questionable, the unspecific stimulation of innate immunity by IAV DIPs may be advantageous; in particular, regarding a potential universal efficacy against such new (and future) variants.”
These studies showed that the cell culture-derived IAV DIPs are highly potent inhibitors of SARS-CoV-2 replication in human lung cells. Based on this study's results, the researchers suggested an unspecific stimulation of the innate immunity by IAV DIPs to suppress the SARS-CoV-2 replication.
In addition to vaccination, the IAV DIPs represent an exciting option for prophylactic treatment. It can boost antiviral immunity in a person at acute risk for a viral infection. It is an effective option for treatment during an early phase post-infection and may prevent fatal COVID-19 outcomes.
“We propose IAV DIPs as effective antiviral agents for the treatment of COVID-19 and, potentially as universal antiviral agents not only against different influenza subtypes but also against other (including newly emerging) IFN-sensitive respiratory viruses.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Antiviral activity of influenza A virus defective interfering particles against SARS-CoV-2 replication in vitro through stimulation of innate immunity, U. Rand, S.Y. Kupke, H. Shkarlet, M.D. Hein, T. Hirsch, P. Marichal-Gallardo, L. Cicin-Sain, Udo Reichl, D. Bruder, bioRxiv 2021.02.19.431972; doi: https://doi.org/10.1101/2021.02.19.431972, https://www.biorxiv.org/content/10.1101/2021.02.19.431972v1
- Peer reviewed and published scientific report.
Rand, Ulfert, Sascha Young Kupke, Hanna Shkarlet, Marc Dominique Hein, Tatjana Hirsch, Pavel Marichal-Gallardo, Luka Cicin-Sain, Udo Reichl, and Dunja Bruder. 2021. “Antiviral Activity of Influenza a Virus Defective Interfering Particles against SARS-CoV-2 Replication in Vitro through Stimulation of Innate Immunity.” Cells 10 (7): 1756. https://doi.org/10.3390/cells10071756. https://www.mdpi.com/2073-4409/10/7/1756.