Researchers in the United States say the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant – P.1 – that has recently emerged in Brazil shows a worrying level of resistance to neutralization by antibodies.
The SARS-CoV-2 virus is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that poses an ongoing threat to mankind and has now claimed the lives of more than 2.56 million people globally.
The team from Columbia University in New York found that as well as being resistant to several neutralizing monoclonal antibodies (mAbs), the P.1 variant is also over six times more resistant to neutralization by convalescent plasma and more than twice as resistant to sera from vaccinees than the wildtype virus.
However, the loss of neutralizing activity that convalescent plasma and vaccine sera showed against P.1 was not as significant as the reported loss of activity against the B.1.351 variant identified in South Africa.
David Ho and colleagues say this suggests that the risk of re-infection and decreased vaccine efficacy posed by P.1 may not be as severe as that posed by B.1.351.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Recent variants exhibit resistance to neutralization
A number of studies have shown that the SARS-CoV-2 variants B.1.1.7 and B.1351 recently identified in the UK and South Africa, respectively. These harbor mutations confer resistance to the neutralizing activity induced by previous infection or vaccination.
The P.1 variant that has emerged in Northern Brazil has been shown to contain ten mutations in the viral spike protein – the main structure SARS-CoV-2 uses to bind to and infect cells.
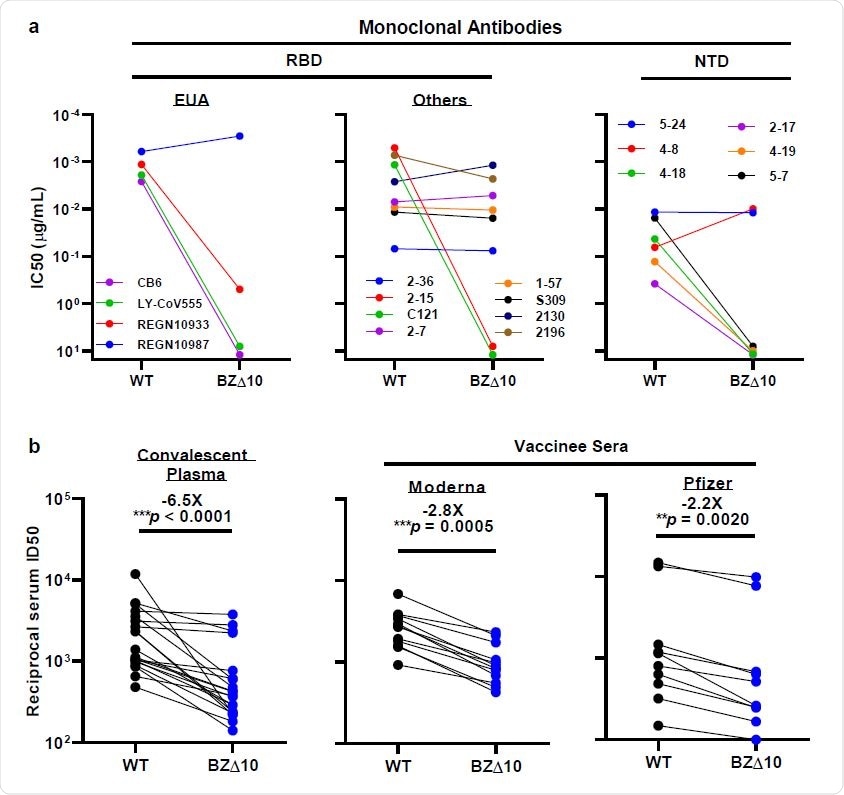
Neutralization of WT and BZ△10 pseudoviruses by mAbs, convalescent 178 plasma, and vaccinee sera. a, Changes in neutralization IC50 of select RBD and NTD mAbs. b, Changes in reciprocal plasma neutralization ID50 values of convalescent plasma and reciprocal serum ID50 values for persons who received Moderna or Pfizer vaccine. Mean fold change in ID50 relative to the WT is written above the p values.
As well as the well-established D614G mutation that became dominant early on in the pandemic, P.1 contains three mutations (K417T, E484K, and N501Y) in the spike’s receptor bind domain (RBD), five mutations (L18F, T20N, P26S, D138Y, and R190S) in the N-terminal domain (NTD), and one mutation (H655Y) near the furin cleavage site.
The three RBD mutations are the same as those found in the RBD of B.1.351, a variant that has been shown to resist neutralization by some mAbs, convalescent plasma, and sera from vaccines.
“This new variant could threaten the efficacy of current mAb therapies or vaccines because it shares mutations at the same three RBD residues with B.1.351,” writes Ho and the team.
What did the researchers do?
The researchers created a SARS-CoV-2 pseudovirus containing all 10 mutations (BZ∆10) found in the P.1 variant and assessed its susceptibility to neutralization by 18 neutralizing mAbs, 20 plasma samples from convalescent individuals and 22 serum samples from vaccinees.
When the team tested BZ∆10 against four mAbs that have received emergency use authorization (EUA), including imdevimab, casirivimab, bamlanivimab, and etesevimab, the only one that retained its original neutralizing activity was imdevimab. The neutralizing activity of the remaining three was either markedly reduced or undetectable.
“Here we report that P.1 is indeed resistant to neutralization by several RBD-directed mAbs, including three with EUA,” write Ho and colleagues.
mAbs targeting the RBD and NTD
Next, the team tested eight RBD-targeting mAbs, which revealed that two previously potent mAbs exhibited no neutralizing activity against BZ∆10.
“Overall, these findings mimic those observed for B.1.3513, which should not be surprising since the triple RBD mutations in P.1 and B.1.351 are largely the same,” say the researchers.
The BZ∆10 pseudovirus was also highly resistant to neutralization by four of six NTD-targeting mAbs tested. However, two mAbs targeting the antigenic supersite in NTD that have completely lost neutralizing activity against B.1.3513, remained active against BZ∆10.
“The resistance profiles are markedly different for P.1 and B.1.351, reflecting their distinct sets of mutations in NTD,” writes the team.
To understand the specific mutations responsible for this pattern of neutralization, the researchers tested these NTD-targeting mAbs against a panel of pseudoviruses that each contained only one of the five NTD mutations (L18F, T20N, P26S, D138Y, and R190S) found in P.1.
Unsurprisingly, the two mAbs that remained active against BZ∆10, retained neutralizing activity against all single-mutation pseudoviruses. Of the four remaining mAbs, one or more of the five mutations accounted for the loss of neutralizing activity against BZ∆10.
What about convalescent plasma and vaccinated sera?
When the researchers tested 20 convalescent plasma samples for neutralization activity against BZ∆10, they observed a 6.5-fold reduction in neutralizing activity against BZ∆10, compared with against wildtype pseudoviruses.
Finally, serum samples from 12 recipients of the Moderna mRNA-1273 vaccine and 10 recipients of the Pfizer BNT162b2 vaccine were assayed for neutralization against BZ∆10 and wildtype pseudoviruses.
A reduction in neutralization activity against BZ∆10 was observed for every sample, but the magnitude of the loss was modest (2.8-fold for Moderna; 2.2 fold for Pfizer), compared with the loss against B.1.351 pseudovirus (8.6 fold for Moderna; 6.5 fold for Pfizer).
What do the authors suggest?
“Both convalescent plasma and vaccinee sera show a significant loss of neutralizing activity against P.1, but the diminution is not as great as that reported against B.1.351,” say the researchers. “Therefore, the threat of increased re-infection or decreased vaccine protection posed by P.1 may not be as severe as B.1.351.”
The team also says that since the RBD mutations are primarily the same between the two variants, the difference in their neutralization susceptibility to sera suggests that NTD mutations may significantly affect the susceptibility of SARS-CoV-2 to antibody neutralization.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources