The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected hundreds of millions worldwide since its emergence more than a year ago. The virus has also been mutating throughout the pandemic, with several new variants, likely more infectious, being reported over the last few months.
To combat SARS-CoV-2, several vaccines have now been approved, and more than 100 are at different stages of clinical trial. However, the approved vaccines have shown a reduced efficacy against the new variants, which have mutations on the virus spike protein, N-terminal domain, and other regions.
The B.1.351 variant appears to be more resistant to convalescent sera and sera from vaccinated people compared to the B.1.1.7 variant. This suggests the need to develop vaccines with a broad neutralizing antibody response against different variants.
Broad antibody responses need long-lived germinal center (GC) reactions to activate precursor B cells and form long-term immune memory. Long-lived GC reactions can be maintained by antigen retention in lymph node follicles, which can be used to develop broad antibody response vaccines.
Previously, researchers designed a spike protein on three self-assembling protein nanoparticles as SARS-CoV-2 vaccine candidates. In a new study published on the bioRxiv* preprint server, researchers tested these against SARS-CoV-2 variants.
Nanoparticle vaccines retained in lymph nodes
The authors found that all three nanoparticle-based vaccines showed higher levels of neutralizing antibodies compared to a control vaccine, by up to 8-fold, in mice sera. One of the three also showed higher antibodies against the new variants: 0.5-fold for B.1.1.7, 0.8-fold for B.1.351, and 1.8-fold for P.1, compared to the Wuhan-1 strain. All three variant pseudoparticles were neutralized by the mouse sera.
The team found that the route of injection also affected antibody levels. When the vaccines were injected intradermally on the mouse footpads, the vaccines that had about 20 spikes gave higher neutralizing antibody levels than the soluble version. This suggests a higher nanoparticle display is necessary for eliciting a broad antibody response.
To understand why the nanoparticle spikes show better performance than the soluble spikes, the researchers tested the soluble and nanoparticle vaccines in mice by injecting them into footpads. Upon analyzing the lymph nodes, they found that the nanoparticles-based spikes accumulated in the lymph node follicles, with their distribution in them differing slightly based on the number of doses.
The control vaccine entered lymph nodes within two hours and were gone by 48 hours. However, the two large nanoparticle vaccines entered the follicles about 12 hours after injection and were detected up to two weeks, indicating greater retention than the control. This is similar to previous studies that reported smaller nanoparticles cleared quickly while large nanoparticles were retained for a longer time.
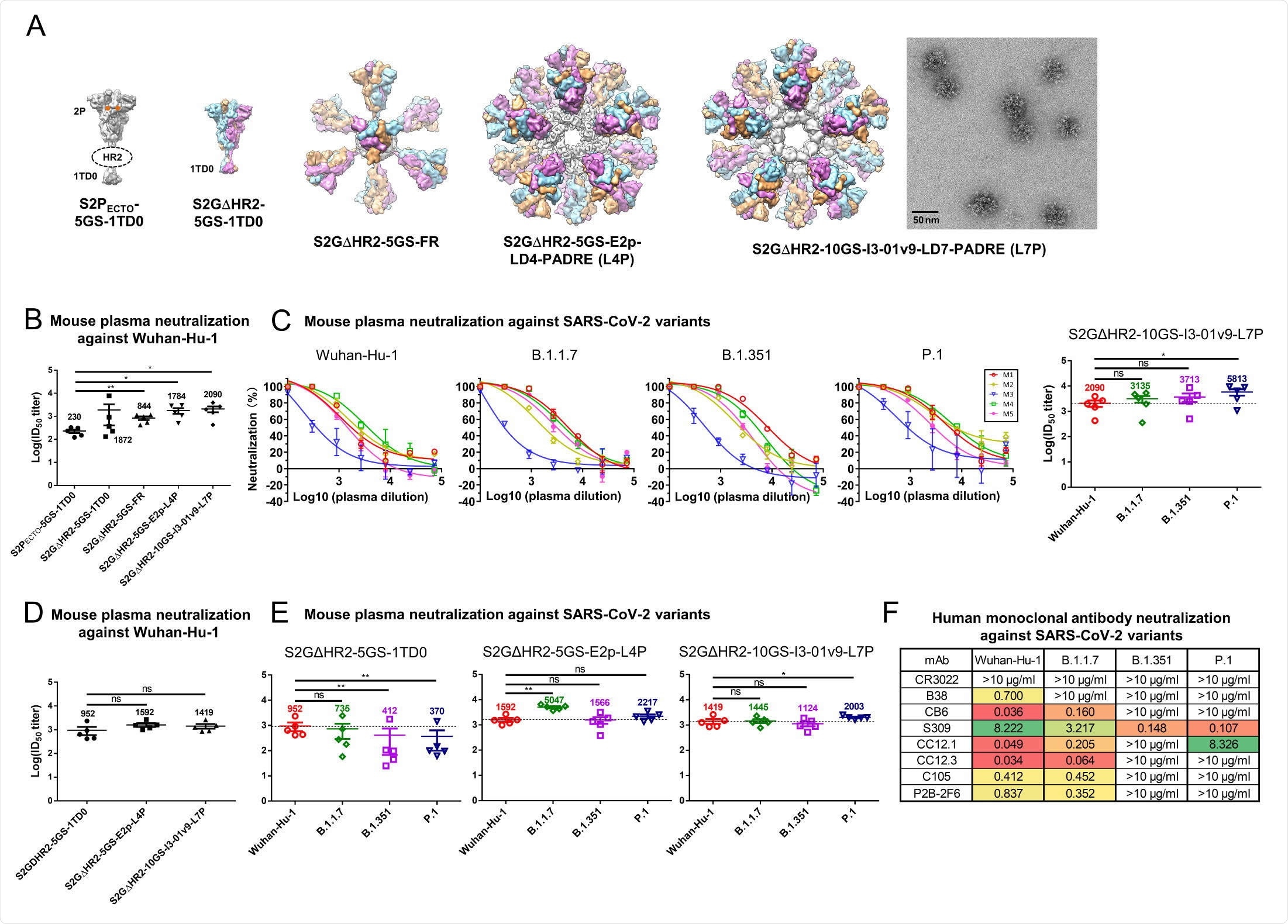
SARS-CoV-2 SApNP vaccines induce broadly neutralizing antibody responses to three variants of concern. (A) Molecular surface representations of vaccine constructs, including two spikes (S2PECTO-5GS-1TD0 and S2GΔHR2-5GS-1TD0) and three spike presenting SApNPs (S2GΔHR2-5GS-ferritin (FR), S2GΔHR2-5GS-E2p-LD4-PADRE (E2p687 L4P), and S2GΔHR2-10GS-I3-01v9-LD7-PADRE (I3-01v9-L7P)). Representative negative stain EM (nsEM) image of S2GΔHR2-10GS- I3-01v9-L7P SApNPs is shown on the right. (B) Neutralization of the original Wuhan-Hu-1 strain by mouse plasma induced by 5 different vaccines at week 5 after two intraperitoneal injections. ID50 titers derived from SARS-CoV-2-pp neutralization assays are plotted, with average ID50 values labeled on the plots. (C) Mouse plasma neutralization against the original Wuhan-Hu-1 strain and three variants, B.1.1.7, B1.351, and P.1, at week 5 after two intraperitoneal injections of the adjuvanted S2GΔHR2-10GS-I3- 01v9-L7P vaccine. Left panels 1-4: percent neutralization plots of individual mice against 4 SARS-CoV-2 strains; Right panel: ID50 plot. In (B) and (C), the plasma samples were generated in the previous study (41), where mice were immunized with 50 μg of adjuvanted vaccine antigen. (D) Neutralization of the original Wuhan-Hu-1 strain by mouse plasma induced the S2GΔHR2 spike and two large S2GΔHR2-presenting SApNPs. Vaccines were administered via footpad injections (0.8 μg/injection, for a total of 3.3 μg/mouse). (E) Mouse plasma neutralization against the original Wuhan-Hu-1 strain and three variants, B.1.1.7, B1.351, and P.1, at week 5 after two footpad injections of the S2GΔHR2 spike and two large S2GΔHR2- presenting SApNPs. In (B)-(E), the ID50 values are plotted as mean ± SEM. The data were analyzed using two-tailed unpaired Student’s t-test for comparison between different vaccine groups or two-tailed paired Student’s t-test for comparison of ID50 titers against SARS-Cov-2 variants using the same plasma samples from a mouse. *p < 0.05, **p < 0.01. (F) Neutralization of four SARS-CoV-2 strains by human monoclonal antibodies including CR3022, B38, CB6, S309, CC12.1, CC12.3, C105, and P2B-2F6. IC50 values are listed and color-coded (white: no neutralization; green to red: low to high). The IC50 values were calculated with the %neutralization range constrained within 0.0-100.0%.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Lymph nodes retain larger particles
Further tests revealed that the follicular dendritic cells (FDCs) play a crucial role in the retention of the vaccine in the lymph nodes. Using transmission electron microscopy, the team looked at the interface between FDCs and B cells. They found intact spike nanoparticles on and in macrophages inside lymph nodes, suggesting FDCs can help retain the spike nanoparticles.
The team also found that the spike nanoparticles vaccines induced strong, long-lived GCs, while the soluble spike did not sustain GCs eight weeks after vaccination, either with a single dose or with a booster dose. The nanoparticles showed up to a five-fold increase in GC B cells and T follicular helper cells compared to the soluble spike vaccine.
The results showed that the nanoparticle vaccine candidates showed six-fold longer retention and four-fold more accumulation in lymph nodes than the soluble spike protein candidate. It is likely this is because of the properties of lymph nodes that help retention of larger particles. The nanoparticle candidates are uniquely suited for producing long-lived GCs in lymph nodes.
Although protein vaccines have long been used with reasonable safety and efficacy, they have yet to be used for SARS-CoV-2. Protein vaccines alone or as boosters for nucleic acid vaccines. The team found that the spike nanoparticle vaccines adjuvanted with AddaVax and aluminum phosphate induced stronger GC reactions than vaccines without adjuvants. Further testing of adjuvants and a broader understanding of how nanoparticle vaccines and other platforms behave in the body will help develop vaccines more quickly.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhang, Y.-N. et al. (2021) Mechanism of a COVID-19 nanoparticle vaccine candidate that elicits a broadly neutralizing antibody response to SARS-CoV-2 variants, bioRxiv, https://doi.org/10.1101/2021.03.26.437274, https://www.biorxiv.org/content/10.1101/2021.03.26.437274v1
- Peer reviewed and published scientific report.
Zhang, Yi-Nan, Jennifer Paynter, Cindy Sou, Tatiana Fourfouris, Ying Wang, Ciril Abraham, Timothy Ngo, Yi Zhang, Linling He, and Jiang Zhu. 2021. “Mechanism of a COVID-19 Nanoparticle Vaccine Candidate That Elicits a Broadly Neutralizing Antibody Response to SARS-CoV-2 Variants.” Science Advances 7 (43). https://doi.org/10.1126/sciadv.abj3107. https://www.science.org/doi/10.1126/sciadv.abj3107.