Researchers in Israel have shown that three national programs established by the Israeli government have successfully reduced cases of severe coronavirus disease 2019 (COVID-19) and death caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
The study revealed that despite the increased transmissibility of the SARS-CoV-2 variant B.1.1.7, mass deployment of reverse-transcriptase polymerase chain reaction (RT-PCR) testing, proactive surveillance in nursing homes and prioritized immunization with the Pfizer-BioNTech BNT162b2 vaccine have decreased viral transmission, severe illness, and deaths in Israel.
“Pro-active protection programs such as routine surveillance and monitoring of populations at risk combined with prioritized vaccination are achievable and will reduce severe illness and subsequent death,” writes the team from Tel Aviv University and Electra-TAU laboratory in Omer.
A preprint version of the study is available while it completes peer review, and the final article will be published in the journal Cell Reports Medicine.
Spread of B.1.1.7 SARS-CoV-2 variant in Israel
More about recently emerged variants
Since the novel SARS-CoV-2 virus was first identified in Wuhan, China, in late December 2019, several variants have emerged that confer increased infectivity and transmission.
The B.1.1.7 variant, which emerged in England during December 2020, contains at least three mutations that have been shown to increase infectivity and transmission.
Preliminary evidence from epidemiological studies in England estimated that the B.1.1.7 variant is between 43% and 82% more transmissible than the original wild-type virus and has increased the effective reproduction number by a factor of 1.40 to 1.88.
“Nevertheless, increased transmissibility for the B.1.1.7 in real life outside of the UK is still unclear,” says Ariel Munitz and colleagues.
In addition, interventions that may slow down the spread of the B.1.1.7 variant remain to be determined, they add.
What did the current study involve?
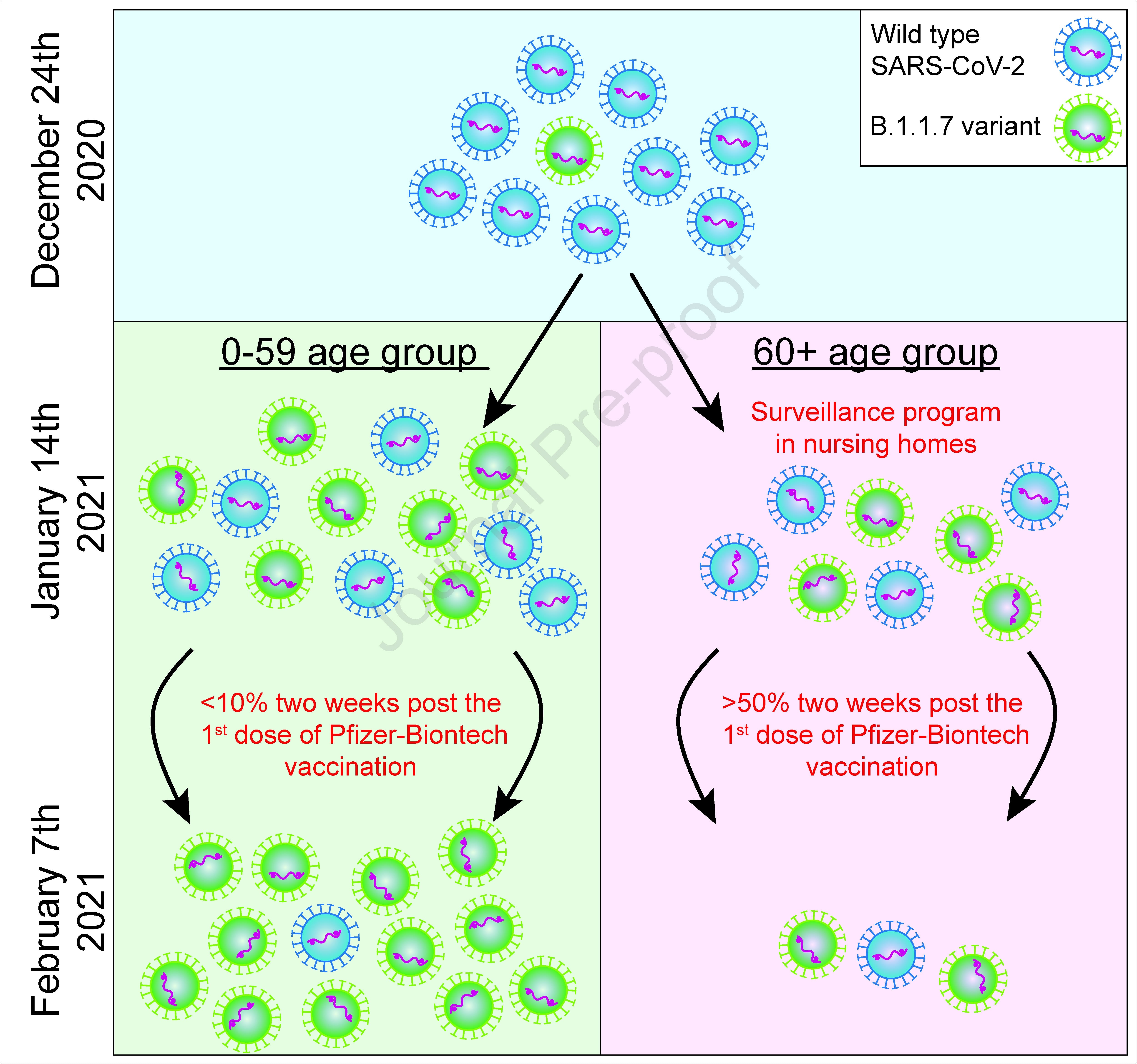
During the COVID-19 pandemic, the Israeli government established three national protection programs. Firstly, mass deployment of RT-PCR testing provided up to 9,200 tests per million inhabitants per day. Secondly, an ongoing surveillance testing program was set up in nursing homes. Thirdly, a nationwide rollout of the Pfizer-BioNTech BNT162b2 vaccine was initiated.
To investigate the transmission capability of the B.1.1.7 variant and to determine the impact of the three national programs, Munitz and colleagues analyzed data available on 292,000 RT-PCR samples that were collected from December 6th, 2020, until February 10th, 2021.
“This timely data provides an ideal setting to understand the dynamics of the dominant B.1.1.7 variant in light of disease surveillance and vaccination programs,” writes the team.
What did the study find?
Until December 22nd, the B.1.1.7 variant was not detected within the pool of positive cases in Israel.
However, the variant had become the dominant strain in Israel within 3.5 weeks (by January 19th, 2021) and was identified in more than 90% of positive tests within six weeks (by February 4th).
The study revealed that the B.1.1.7 variant is 45% more transmissible than the original wild-type strain.
The team says this result is within the range of previous estimates, which pointed to a 56% increase in transmissibility in England.
The impact of active surveillance in nursing homes
Next, the team demonstrated that routine active surveillance programs in nursing homes significantly reduced transmission of the B.1.1.7 variant.
The programs involve nursing home residents being tested approximately every three days, irrespective of any potential exposure to infection. By contrast, community testing is only mainly performed following symptom presentation or contact with confirmed COVID-19 patients.
The researchers, therefore, compared the RT-PCR cycle threshold (Ct) values in people aged 60 plus living in nursing homes with those of age-matched individuals living in the general community.
The Ct value is a predictive marker of viral load, where a higher number indicates a lower viral load.
The analysis revealed that the Ct values in nursing homes were significantly higher than in the age-matched community population.
“The higher Ct values in nursing homes is likely due to the ongoing surveillance program in nursing homes,” writes the team. “Since increased viral load drives transmission, our data underscores the important role of random surveillance testing in nursing homes and other high-risk communities.”
The impact of the Pfizer-BioNTech vaccine
Importantly, a sharp decline in cases of new infections was observed once about half of the elderly population had been protected by a first vaccine dose for two weeks.
“Our data demonstrate a clear containment of the B.1.1.7 variant by the Pfizer-BioNTech vaccine in the 60+ age group,” writes the team.
The researchers say this interpretation is supported by the relative rise in B.1.1.7 transmission in people aged 0 to 59 years and the fact that aside from the vaccination regime, no other intervention measures were introduced in that age group at that time.
What do the authors conclude?
The team says the data are among the first to report real-world data of vaccination efficacy in a large community cohort.
“Collectively our data show that proactive surveillance combined with prioritized vaccination are achievable and can lead to decreased transmission, severe illness and subsequent death,” says Munitz and colleagues.
However, “despite these data, the efficiency of the vaccine should be monitored over time in order to conclude that long protection is achievable,” they warn.
Journal reference:
- Munitz A, et al. BNT162b2 Vaccination Effectively Prevents the Rapid Rise of SARS-CoV-2 Variant B.1.1.7 in high risk populations in Israel. Cell Reports Medicine, 2021. DOI: https://doi.org/10.1016/j.xcrm.2021.100264