
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
With the total number of confirmed global cases of COVID-19 surpassing 330 million, including over 5.54 million deaths, there remains an urgent need for effective treatment methods.
In the early months of the COVID-19 pandemic, the United States Food and Drug Administration (FDA) provided emergency use authorization (EUA) for the use of HCQ and CQ in the treatment of hospitalized COVID-19 patients. However, most retrospective-observational studies of HCQ/CQ in hospitalized COVID-19 patients provide no evidence supporting the efficacy of this treatment.
About the study
The current study conducted an individual participant data (IPD) meta-analysis to determine the efficacy and safety of HCQ and CQ in the treatment of hospitalized COVID-19 patients overall and in particular cohorts of interest.
The study included U.S.-based randomized clinical trials (RCTs) of HCQ/CQ to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients. The researchers analyzed eight RCTs in 770 hospitalized COVID-19 patients and compared HCQ/CQ and control treatment.
While all RCTs had an HCQ treatment arm, one study had CQ as an additional treatment arm. The comparators were placebo in three trials, azithromycin in two trials, and standard/usual care in two trials.
The study with the CQ treatment arm compared HCQ and CQ with and without azithromycin. HCQ dosing was usually 400 mg orally twice daily on day one and 200 mg twice daily on days two to five; thus, the total amount of HCQ administered was 2,400 mg. The study also included three blinded trials and open-label trials.
Clinical improvement in patients was measured as a primary outcome using a seven-point ordinal scale with the following levels: (1) death; (2) hospitalized, on mechanical ventilation or extracorporeal membrane oxygenation (ECMO); (3) hospitalized, on non-invasive ventilation (BiPAP/CPAP and/or high-flow oxygen); (4) hospitalized, requiring oxygen; (5) hospitalized, not requiring oxygen; (6) not hospitalized, with limitation; (7) not hospitalized, without limitations. The outcomes were measured for a period of 28-35 days post-enrollment.
The study included subgroups classified by age comprised of individuals aged 29 years or younger, between 30-49 years, between 50-69 years, between 70-79 years, and 80 years and older. Additionally, subgroups were classified according to disease severity and comorbidities including asthma, smoking, vaping, chronic obstructive pulmonary disease, liver disease, hypertension, myocardial infarction, congestive heart failure, cerebrovascular disease, tumor, dementia, and diabetes.
Study findings
Out of the 770 patients with laboratory-confirmed SARS-CoV-2 infection included in the present study, 412 were randomized to HCQ/CQ treatment and 358 in the control group. The researchers concluded that the effect of HCQ/CQ was independent of body mass index (BMI) or baseline ordinal score within all the RCTs.
In this study, the standardized proportional odds ratio (OR) at 28-35 days was 0.97 and the corresponding unadjusted proportional OR was 0.98. These results show that HCQ/CQ had no effect on hospitalized COVID-19 patients.
The mortality rate at 28-35 days was found to be similar for both HCQ/CQ and control groups. A median post-enrollment hospital length of stay of seven days was observed for the HCQ/CQ and control groups.
The overall rates of adverse effects (AEs) and serious adverse events (SAEs) were significantly higher in the HCQ/CQ as compared to the control group. The rate of liver function tests (LFTs) elevation was also higher in the HCQ/CQ group as compared to the control groups. The AE and SAE rates of Q-T corrected (QTc) prolongation and arrhythmia were similar in the HCQ/CQ and control groups.
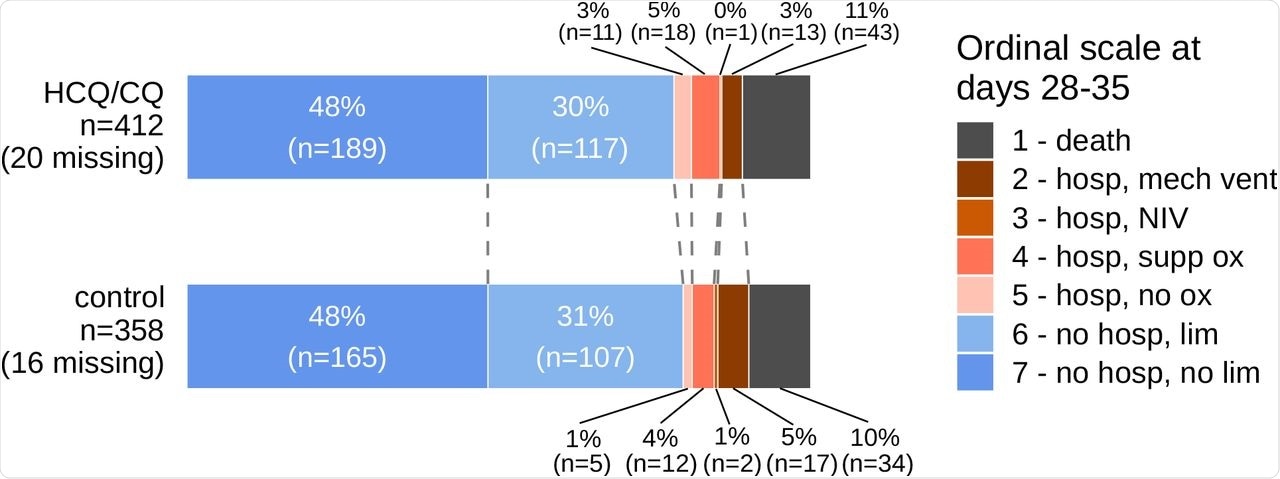
Primary Outcome Data by Treatment Group.
Conclusions
The researchers concluded that the results of this analysis are concordant with that of at least 50 previously published meta-analyses evaluating HCQ/CQ in hospitalized COVID-19 patients. None of the subgroups included in the study particularly benefited from the HCQ/CQ treatment. A higher increase in the overall rates of AE and SAEs, along with LFT elevation, was observed in the HCQ/CQ groups than in the control groups.
With no evidence of the efficacy of HCQ/CQ in COVID-19 treatment, no difference in mortality rates as compared to control groups, and significantly higher AEs and SAEs, the authors concluded that HCQ/CQ was not effective for the treatment of COVID-19 in hospitalized patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Di Stefano, L., Ogburn, E. L., Ram, M., et al. (2022). Hydroxychloroquine/Chloroquine for the Treatment of Hospitalized Patients with COVID-19: An Individual Participant Data Meta-Analysis. medRxiv. doi:10.1101/2022.01.10.22269008. https://www.medrxiv.org/content/10.1101/2022.01.10.22269008v1.
- Peer reviewed and published scientific report.
Di Stefano, Leon, Elizabeth L. Ogburn, Malathi Ram, Daniel O. Scharfstein, Tianjing Li, Preeti Khanal, Sheriza N. Baksh, et al. 2022. “Hydroxychloroquine/Chloroquine for the Treatment of Hospitalized Patients with COVID-19: An Individual Participant Data Meta-Analysis.” Edited by A. M. Abd El-Aty. PLOS ONE 17 (9): e0273526. https://doi.org/10.1371/journal.pone.0273526. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0273526.