New research reveals that social isolation and stress during the COVID-19 pandemic accelerated the aging of the brains of older adults, regardless of whether they contracted the virus, highlighting the urgent need for protective strategies beyond infection control.
 Study: Accelerated brain ageing during the COVID-19 pandemic. Image Credit: Shyntartanya / Shuttertock
Study: Accelerated brain ageing during the COVID-19 pandemic. Image Credit: Shyntartanya / Shuttertock
In a recent study published in the journal Nature Communications, a group of researchers tested whether the coronavirus disease 2019 (COVID-19) pandemic, with or without severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, accelerated biological brain aging in middle-aged and older adults.
The study sought to distinguish the impact of infection itself from the effects of pandemic-related social disruption on brain aging.
Background
Every three seconds, someone worldwide develops dementia, yet scientists still debate what hastens cerebral aging. The advent of COVID-19 raised concerns, as SARS-CoV-2 affects both gray matter (GM) and white matter (WM) on magnetic resonance imaging (MRI). Yet millions who never caught the virus endured isolation and missed care during lockdowns, stressors that also erode brain resilience.
Cross-sectional snapshots lack pre-pandemic baselines, making it impossible to distinguish between biological and social damage. Identifying the primary driver is crucial for predicting dementia and informing protective policies. The complexity of mental health outcomes during the pandemic, including variable findings across populations, has also made interpretation challenging.
Further research is needed to clarify causality and identify modifiable risk factors for aging populations worldwide.
About the study
Investigators mined the United Kingdom Biobank (UKBB), a cohort with multimodal MRI. Separate GM and WM brain age models were trained on 15,334 healthy adults scanned before March 2020. From 1,865 imaging-derived phenotypes (IDPs) summarizing structure, diffusion, and T2 signals, principal component analysis (PCA) extracted 50 components. Elastic net regression accurately predicted chronological age, yielding a mean absolute error (MAE) of nearly three years; test-retest reliability was demonstrated by an intraclass correlation coefficient (ICC) of 0.98.
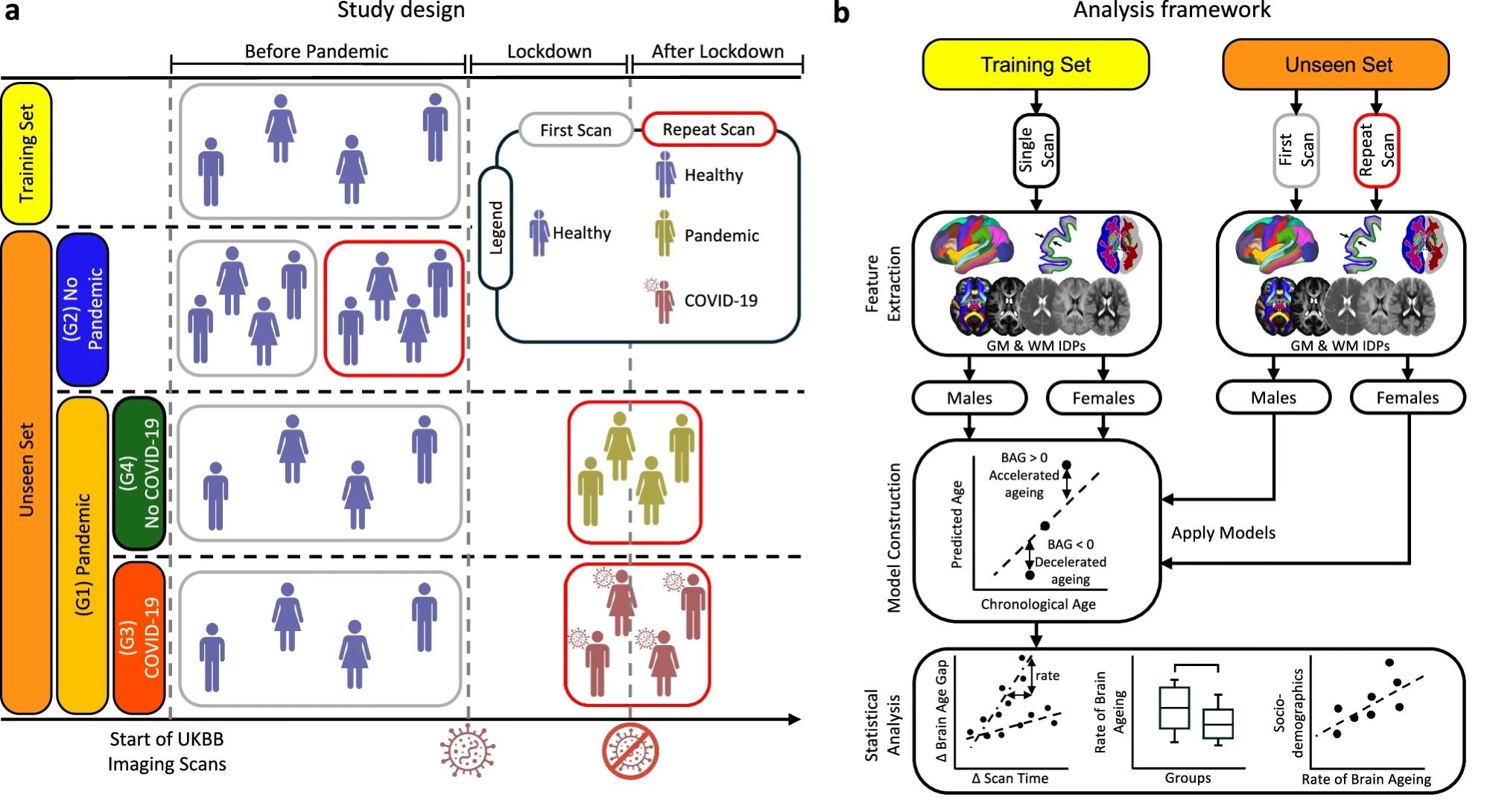 a A brain age prediction model was trained using 20-fold cross-validation on healthy participants with a single pre-pandemic scan (training set). The model was applied to an unseen set comprising the Pandemic group (G1) and the No Pandemic group (G2). G1 was further subdivided into Pandemic–COVID-19 (G3) and Pandemic–No COVID-19 (G4). b Imaging-derived phenotypes (IDPs) were extracted from grey matter (GM) and white matter (WM) across scan times. Separate prediction models were trained by tissue type and sex using pre-pandemic data, and then applied independently to scans from different time points to estimate brain age gap (BAG). Statistical analyses assessed pandemic- and infection-related effects using longitudinal data.
a A brain age prediction model was trained using 20-fold cross-validation on healthy participants with a single pre-pandemic scan (training set). The model was applied to an unseen set comprising the Pandemic group (G1) and the No Pandemic group (G2). G1 was further subdivided into Pandemic–COVID-19 (G3) and Pandemic–No COVID-19 (G4). b Imaging-derived phenotypes (IDPs) were extracted from grey matter (GM) and white matter (WM) across scan times. Separate prediction models were trained by tissue type and sex using pre-pandemic data, and then applied independently to scans from different time points to estimate brain age gap (BAG). Statistical analyses assessed pandemic- and infection-related effects using longitudinal data.
The models were applied to 996 volunteers, who underwent two MRI sessions. Controls (n = 564) were scanned twice before the pandemic, whereas the pandemic cohort (n = 432) underwent one pre- and one post-restriction scan; 134 of them had laboratory-confirmed coronavirus disease 2019. Of the COVID-19 cases, only 5 out of 134 participants (<4%) required hospitalization, indicating that the cohort was almost exclusively comprised of mild cases.
Groups were balanced for demographics, body mass index, vascular risk, education, income, and deprivation score. Only inter-scan intervals ≥ 24 months were accepted to reduce noise. An adjustment for the inter-scan interval was incorporated to minimize confounding by time between scans.
The primary endpoint was the rate of change in brain age gap (RBAG), calculated as RBAG = ΔBAG / Δtime (months per year). Permutation-based analysis of variance with false discovery rate (FDR) correction was used to test the effects of pandemic exposure, infection status, sex, chronological age, and socioeconomic adversity. All MRI acquisitions used identical Siemens scanners and standardized protocols across four sites.
Study results
The predicted brain age at the first MRI session was statistically indistinguishable among the three cohorts, including pre-pandemic controls, pandemic participants without SARS-CoV-2, and pandemic participants with laboratory-confirmed COVID-19, confirming a balanced baseline neuroanatomy. When the same volunteers returned a median of 2.7 years later, the brains of people who had lived through lockdowns appeared to be more advanced in age.
After adjusting for inter-scan interval, sex, body mass index, and cardiovascular risk, their brains looked 5.5 months older than those of controls, yielding Cohen d values of 0.61 for GM and 0.70 for WM.
Importantly, SARS-CoV-2 infection itself did not account for the surge in cases. Pandemic volunteers who never caught the virus aged at the same pace as their infected peers, and both subgroups differed significantly from controls once FDR correction was applied.
Chronological age amplified vulnerability, as in controls, with each birthday adding about three brain age days. However, under pandemic conditions, the slope more than doubled to seven days in GM and eight days in WM; the number of infected individuals increased to nearly ten days per calendar year. As the vast majority of infections were mild, the study could not robustly assess whether disease severity (e.g., hospitalization or symptom duration) modulated RBAG.
Sex differences were evident, as under identical pandemic exposure, male brains aged 33% faster than female brains in GM; for WM, the difference was similar in pattern but did not reach statistical significance. The interaction for sex was significant for GM (FDR p = 0.008), but not for WM.
Socioeconomic adversity further widened the gap. Participants reporting low employment security, poor self-rated health, or low household income accumulated between 1.5 and 5.8 additional months of brain aging during follow-up relative to the most advantaged peers, and interaction terms between deprivation indices and pandemic status remained significant.
Cognitive change was selective rather than global. Standard memory and language scores stayed stable across groups, yet only volunteers who experienced COVID-19 displayed slower Trail Making Test (TMT) performance.
Completion times increased by 6–9% for numeric (TMT A) and alternating alphanumeric (TMT B) versions, and higher RBAG correlated with slower TMT A speed (r = 0.23, p = 0.004), indicating that infection-associated neuroinflammation may potentially convert silent structural vulnerability into measurable executive decline.
It should be noted, however, that the study demonstrates association rather than proven causation in this mechanistic link.
BAG estimates remained orthogonal to chronological age; the test-retest ICC stayed 0.98; and MAE for age prediction never exceeded 3.1 years. Mixed effects models that simultaneously entered deprivation, sex, infection status, and chronological age still attributed a substantial proportion of explained RBAG variance to pandemic exposure (approximately 46%, as per supplementary data), underscoring the dominant role of social disruption.
Continued follow-up will reveal whether the observed structural shifts endure or reverse as normal social activities resume.
The study authors note that longer-term follow-up and additional data, including more direct measures of psychological stress, are needed to clarify the persistence and mechanisms of these effects.
Conclusions
To summarize, the COVID-19 era accelerated biological brain aging by roughly half a year in under three calendar years, irrespective of SARS-CoV-2 infection. Older age, male sex, and socioeconomic disadvantage heightened vulnerability, while measurable cognitive decline emerged only when infection layered neuroinflammatory stress atop socially driven tissue changes.
Importantly, these findings should be interpreted in the context of certain limitations, including the predominance of mild COVID-19 cases in the cohort and the lack of pandemic-period mental health data.
These results indicate that protecting brain health in future crises will require more than just infection control; strategies must also mitigate the effects of isolation, unemployment, and healthcare disruptions to prevent silent neural aging and, ultimately, dementia. Community outreach and mental health care play a crucial role in reinforcing resilience.
Journal reference:
- Mohammadi Nejad, A.R., Craig, M., Cox, E.F., Chen, X., Jenkins, R.G., Francis, S., Sotiropoulos, S.N., & Auer, D.P. (2025). Accelerated brain ageing during the COVID-19 pandemic. Nat Commun. 16. DOI: 10.1038/s41467-025-61033-4, https://www.nature.com/articles/s41467-025-61033-4