My name is Jacob Bobonis, and the goal of my doctorate in the lab of Dr. Nassos Typas at the European Molecular Biology Laboratory (EMBL) in Heidelberg was to find the biological function of a mysterious group of bacterial genetic elements called retrons.
What are 'retrons', and what characteristics make them challenging to study?
Retrons were discovered back in the 1980s in bacteria due to their ability to produce massive amounts of small single-stranded DNA in vivo. Early work showed how this small DNA, termed multicopy single-stranded DNA (msDNA), is generated by dedicated reverse transcriptases and small RNAs encoded by retrons, but the function of msDNA and retrons remained a mystery for almost four decades.
The major challenge in finding their function was that retrons seemed non-essential for bacteria, but as happens very often, people were not looking in the right place.

Image Credit: Peshkova/Shutterstock.com
In your latest research, you have discovered a new feature of retrons. Please can you tell us more about how you carried out your study and what you discovered?
Our study details the discovery of how a retron affects the fitness of a pathogen (Salmonella) and how we used this phenotype to discover that retrons are growth-inhibition switches that use their msDNA to sense the presence of viruses (phages).
We showed that retrons contain toxins, which are typically kept inactive by both the reverse transcriptase and msDNA, but specific phage proteins can directly activate these retron toxins, which then inhibit the growth of Salmonella. This self-targeting toxicity renders the phage-infected bacterium unable to produce more phages, thus protecting the bacterial population from viral catastrophe.
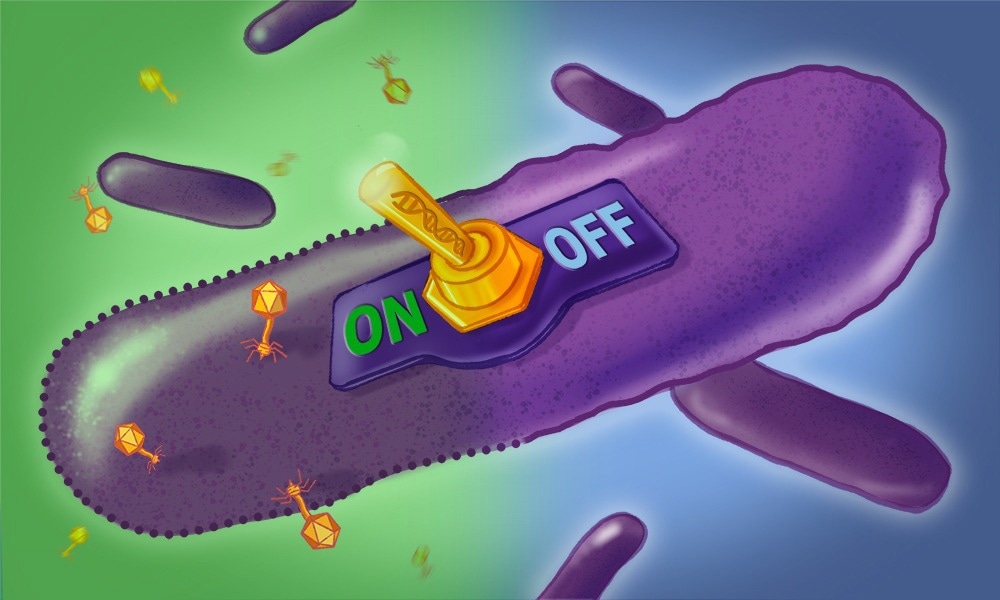
Image Credit: Creative Team/ EMBL
What implications could your research have for the field of immunology? How could using these switches help to treat bacterial infections?
In contrast to common knowledge, our research points to the existence of a highly specialized and elegant prokaryotic antiviral immune system. Bacteria have hundreds of growth-inhibition switches that work like retrons, collectively called toxin-antitoxin systems, but for decades researchers could not find what activates them.
We discovered the first of such triggering mechanisms for retrons and found that they sense epigenetic signals from phages by using their msDNA. This suggests that toxin-antitoxin systems sense highly specific cues from viruses, which, if understood, can be harnessed in multiple creative ways as a new way to inhibit the growth of pathogens by activating these internal growth-inhibition switches. By designing an approach to find how retrons are activated, we provide a tool that can be used to find what activates any of the other thousands of toxin-antitoxin systems.
Image Credit: Jarun Ontakrai/Shutterstock.com
With antimicrobial resistance being one of the top 10 global public health threats facing humanity, are you hopeful that your discovery could help us to understand antimicrobial resistance further? What would this mean for global health?
Our research will be invaluable in leading to treatment options alternative to antibiotics. Finding how toxin-antitoxin systems are activated can lead to developing artificially-designed trigger molecules that enter a pathogen and inhibit its growth in vivo. On the other hand, phages themselves can (and are already) starting to be used in clinics to counter bacterial infections.
We discovered that phages carried their own weapons against retrons and built a roadmap of how other researchers can find phage weapons against other toxin-antitoxin systems. This knowledge can lead to designing decorated phages equipped with sufficient counter-mechanisms to defeat the immune systems of bacterial pathogens and clear potential infections.
When investigating these 'switches,' you combined genetics, bioinformatics, and proteomics disciplines. What are the advantages of having a multidisciplinary approach when making new scientific discoveries?
Scientific endeavors are inherently challenging, and often one approach leads quickly to a dead end. The privilege of using multiple approaches allowed us to avoid such dead-ends and thus solve the next step of the puzzle.
Bioinformatics is a relatively new scientific discipline, combining both biology and computer science. What advantages does this have for research? Do you believe that as the life sciences sector continues to evolve, we will see more researchers using bioinformatic tools in their studies?
Today, not using bioinformatic tools is a major setback for any scientific project in biology, and this distinction will certainly become more profound as time goes by. The major advantage is that, just as having a multidisciplinary approach to exploring problems, computational biology gives hints that can directly guide us to crucial experiments.
Image Credit: Anastasiia Cherviak/Shutterstock.com
Your research team was a collaborative effort comprised of different researchers from different institutions. How important was collaboration to your research and the scientific sector as a whole?
Collaborating across (at least) seven different labs from two continents was key to our research efforts' success. Perhaps equally importantly, working with motivated collaborators makes the scientific process more enjoyable, more creative, and more humane.
What is next for you and your research?
I will soon start working as a postdoctoral fellow at the University of Vienna in the lab of Dr. Martin Polz to explore the molecular intricacies of phage-bacteria interactions in the oceans.
Where can readers find more information?
About Dr. Jacob Bobonis
Postdoctoral researcher at the University of Vienna studying phage-bacteria interactions.
Ph.D. graduate from the European Molecular Biology Laboratory (EMBL) in Heidelberg.
Received the 2021 Nat L. Sternberg thesis prize for outstanding Ph.D. work in bacterial molecular biology.