The first fully biological biobot was developed by assembling embryonic heart and skin cells from African frogs, which led to the formation of an organoid structure with cilia. Cilia are tiny hair-like structures present on the mammalian cell surface that play a vital role in locomotion. This cilia-powered organoid was able to move without any external force.
Despite this advancement, concerns remain about the possibility of immunological rejection of this amphibian-based biobot by the human system. To overcome this challenge, the scientists of the current study developed multicellular, self-constructing, fully biological, and motile biobots from adult human bronchial epithelial cells that have been referred to as “anthrobots.”
Development and characterization of anthrobots
Human bronchial epithelial (NHBE) cells were used to develop anthrobots due to their inherent plasticity and the presence of cilia on the cell surface that help them move back and forth. These cells were isolated from donors and incorporated into a 3D scaffolding structure obtained from rat tissues and resembled the human bronchial environment.
The cells multiplied and formed small organoids after two weeks of culture; however, the cilia of the cells were inside these organoids and, as a result, could not participate in locomotion. Subsequently, the chemical composition of the cell culture growth media was altered, which led to the successful development of cilia on the organoid surface.
Despite similar genetic composition, the organoids differed in shape and size, as they were spherical or ellipsoidal in shape with diameters between 30-500 microns. The cilia were either placed across the entire cell surface or clustered in discrete patches.
The size, shape, and placement of cilia on the organoids primarily depend on the location in the matrix where the cells settle, as well as the viscosity of the growth media. Furthermore, the pattern of movement of these organoids was determined by their morphological features. Depending on their shape, size, and cilia placement, individual organoids exhibited distinct motility patterns ranging from tight loops to straight lines and speeds from five to 50 microns per second.
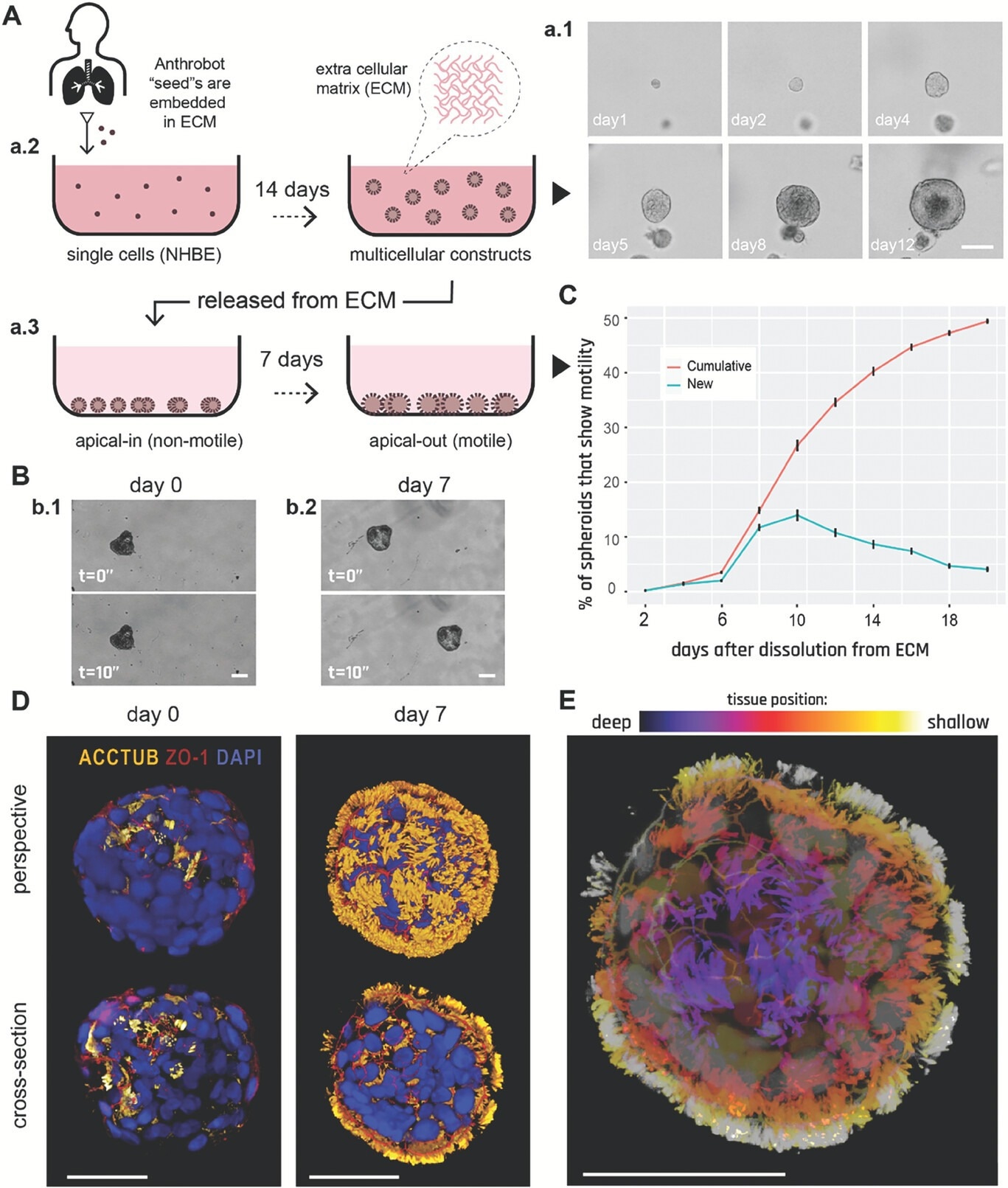
Human bronchial epithelial cells self-construct into multicellular motile living architectures. A) Workflow for producing Anthrobots. NHBE cells’ apical-in to apical-out transition is facilitated by first culturing them in extra cellular matrix (ECM) under appropriate differentiation-inducing conditions, during which time apical-in spheroids self-construct from single cells a.1), and upon the completion of this 14 day period a.2) by releasing mature spheroids from the ECM a.3) and continuing to culture them in low-adhesive environment. B) Phase contrast images of an apical-in b.1) and apical-out b.2) spheroids, captured immediately after dissolution from ECM (day 0) and 7 days after dissolution (day 7), respectively. Day 0 spheroids show no motility, whereas day 7 spheroids show drastically increased motility. C) Percentage of cumulative (total fraction of motile spheroid since day 0) and newly motile spheroids (fraction of motile spheroid that reached motility since the previous time point) in the 3 weeks following dissolution. Out of the 2281 spheroids characterized total, ≈50% consistently showed no signs of motility (despite most having cilia) within this 3-week period and are referred to as non-movers. The data shown on this graph only include the motile bots, N = 1127. D) Immunostaining of two separate spheroids from day 0 and day 7 with a-tubulin (cilia marker), Zonula occludens (ZO)-1 (tight junction marker), and the nuclear stain 4',6-diamidino-2-phenylindole (DAPI). Amount of multiciliate cells on the spheroid surface show a drastic increase by day 7. E) A day 7 Anthrobot with depth information to show full cilia coverage. Bots in panels D,E were immunostained with α-tubulin (cilia marker), ZO-1 (tight junction marker), and DAPI (nuclear stain). Colors represent tissue depth. All scalebars on this figure feature 50 um.
Functional assessment of anthrobots
The scientists explored whether these anthrobots can interact with human cells. For this purpose, a scratch assay, a well-established method to assess wound healing, was performed in human nerve cells.
To this end, human nerve cells were cultured and ‘wounded’ by scratching the cell layer. After that, nerve cells were treated with anthrobots, which subsequently triggered rapid repair of the affected area.
To initiate the wound healing process, “superbot” assemblies were developed by allowing anthrobots to self-aggregate and form large structures. These superbots were placed along the scratch to enable them to span the entire width of the scratch and “bridge” the two sides of the wound like a mechanical stitch. Within 72 hours, a significant regrowth of nerve cells beneath the “superbot bridge” was observed, thereby leading to gap wound healing, as evidenced by the closing of the gap.
Study significance
The current study describes the development and characterization of spheroid-shaped multicellular biological anthrobots with locomotive abilities and tissue repair properties. In the future, the functional ability of these anthrobots can be enhanced by modifying their genome for desired functions, such as drug delivery. Furthermore, these anthrobots can be used for drug screening purposes and understanding drug-disease interactions.
Journal reference:
- Gumuskaya, G., Srivastava, P., Cooper, B. G., et al. (2023). Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells. Advanced Science. doi:10.1002/advs.202303575