Bioabsorbable scaffolds may be considered the new threshold in endovascular treatment. The National Institutes of Health (NIH) report that the most frequent form of heart disease is coronary artery disease (CAD). It is therefore only to be expected that this condition leads the list of causes of death for both men and women in the US.
Endovascular therapy is used to treat CAD, and the emergence of bioresorbable vascular scaffolds (BRS) is often called the next revolution in this field. BRS is used to keep the diseased blood vessel open while it is healing and during the remodeling phase.
Formerly metal stents were used for this purpose, but these must remain within the body to fulfil their purpose. However, with BRS the stent polymer undergoes degradation in the body and is absorbed, but the vessel remains open. This prevents the problem of late loss of vascular lumen and angina. This changeover from metal to polymer stents has taken much time and presented many issues, but the benefits are immense and numerous.
The Development of BRS
Current treatment of coronary artery disease is headed by percutaneous coronary intervention (PCI), or coronary angioplasty. This is a sophisticated and proven method to restore blood flow through coronary arteries that have undergone obstruction.
Many studies based on historical controls have shown that PCI is a vastly better way to treat this condition. One study followed up patients for 15 years after BMS implantation had been carried out, and found that there was a cumulative failure rate at the target lesion (target lesion failure, TLF) at five years of about 23%, while it rose to about 50% at 15 years. These results are shown in Figure 1A.
Another follow-up study followed up patients who had received either of two DES with respect to TLF, and found that the TLF was about 19% for one which released paclitaxel, and 13% for the other which eluted everolimus – the latter is now top in its category.
When a BRS is compared to the above types of DES, it is expected to show the same level of clinical effect at first, but to improve its performance over the course of time, as shown in Figure 1B. That is, it should show TLF which is the best in its class over about 12 months.
From this time onwards until about five years post-implant, BRS-related TLF should reduce dramatically in comparison to DES because of resorption of the stent, as the artery functions more and more normally. If this is confirmed to be so, BRS will have fulfilled its promise.
Many BRS generations have been the subject of short-term studies as well, such as the Absorb III bioresorbable vascular stent (BVS) from Abbott which is undergoing current clinical trials. It is designed as a prospective multicenter single-blinded trial, with 2000 subjects who were given either the Absorb BVS (2:1) or the Xience CoCr-EES. The study is planned to continue for five years and is expected to prove that Absorb BVS produces at least identical results to the other at 1 year, and better results between 1-5 years. The results of the first year were reported in 2015 and were as expected.
Despite the statistical results proving the Abbott device to be non-inferior, overall the Xience device showed better results. For instance, the former showed more scaffold clotting, in particular when the stent was placed in smaller vessels. This could be because of the thickness of the structure, 157 microns, along with improper implantation methods, which are proportionally counterproductive as strut thickness increases.
After implantation, all patients should have post-dilation with a non-compliant balloon that has high pressure. This is not followed in most DES implantation procedures at present, but is essential to make sure the strut fully apposes the vessel wall by completely expanding the scaffold. A potentially improved technique might be to implant the strut directly into the wall or the arterial plaque, so that its effective thickness is reduced.
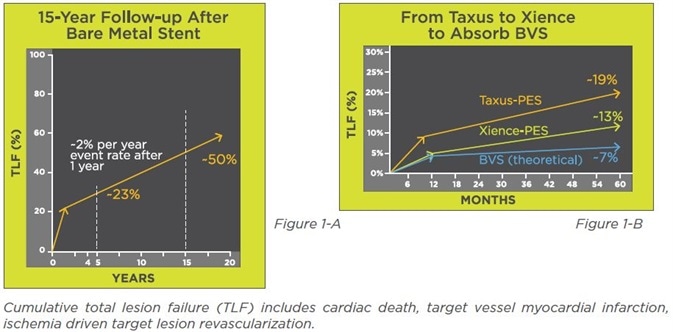
Figure 1. Clinical data showing the development path from bare metal stents to bioresorbable scaffolds.
The Reason Why Strut Thickness is Important
Stent use not only has a risk of recurrent stenosis at the site of the lesion, but also of clot formation. This is admittedly low, but extremely serious in this context. One factor that is associated with thrombosis of the stent is the strut thickness. This is explained as follows: once a stent is implanted, the distal side of the strut shows a major area of recirculation which reduces the endothelial stress in this region. This encourages platelet aggregation and clotting. The use of thinner struts reduces the chances of recirculation and so keeps the stress on the arterial endothelium near normal. This is therefore the objective for next generation BRS.
How to Design the Ideal BRS
Producing an ideal BRS is a complex process because many points must be kept in mind through multiple steps.
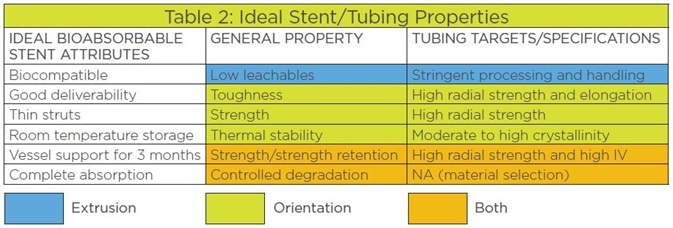
One of the most pressing concerns is the tubing that forms the basis of the BRS. This must have the following properties:
- The percentage of leachables must be low
- It should be strong and also tough
- It should be heat-stable
- It must have good strength retention
- It must have controllable degradation rates
These are represented in Table 2. These properties are responsible for the characteristics of the BRS.
Another very important issue is biocompatibility, which is ensured by using polymers from a single family of standard bioabsorbable polymeric materials. These are mostly polyesters, and have been tried and tested over many years in several medical devices. They also have handling and extrusion processes that are both well-defined and strictly controlled, which means that contamination is avoided, while degradation of the polymer is minimized.
Possessing good deliverability is another important aspect. The scaffold must be capable of both nominal and over-expansion without losing its shape, so that inside the vessel its struts will be well apposed to the wall.
To achieve this, the toughness of the tubing is increased by ensuring that the molecules of the polymer are appropriately oriented, which will bestow good radial strength and elongation. The strut thickness can be reduced if the radial strength is high. If the aim is to achieve a longer shelf life and safe storage at room temperature, the polymer must be processed to a degree of moderate to high crystallinity.
Most specialists agree that the scaffolding should last three months or more, to support the vessel while healing and remodeling proceed at the site of stenting. The strength of the BRS can be adjusted by selecting the right base polymer followed by processing it at a high level of viscosity or molecular weight throughout. This will give the tubing great radial strength.
The period over which the BRS is completely absorbed will also depend upon the selection of the polymer and the molecular weight.
Table 2. The relationship of bioabsorbable stent attributes, bioabsorbable material properties, and the stent tubing specification targets.
However, the use of polymers as substitutes for metals is associated with its own problems, such as the intrinsically lower strength and toughness that characterize polymers. This issue can be resolved only by careful design of struts and scaffolding, as well as the processing of the polymer, since both of these aspects are mutually dependent for their success. Overall, the attributes of the stent and the properties of the stent material form the basis of the requirements specified for the tubing of the BRS.
Obstacles in Processing Polymers Used for BRS
The route to producing a BRS which is suitable for clinical use is complicated, and more than just knowledge of polymers or materials science is involved. The processing and handling of these materials is another essential aspect. One instance is the role played by processing of the polymer during the many steps of manufacture in its final mechanical properties, independent of its chemistry. If either the processing or the handling of the polymer resin during the production stages is substandard, the mechanical integrity and absorption parameters of the material will be less than expected. This will cause unusually high inflammation to result, which will cause the scaffolding to weaken sooner than it should.
About Zeus Inc.
Zeus were among the first to work in the arena of minimally invasive techniques for revolutionary procedures such as neuromodulation. Their PEEK drawn fiber offers a non-metallic replacement for stainless steel. And when a major aircraft manufacturer needed an immediate design change on a harness assembly, Zeus delivered in record time.
Zeus has vast experience in medicine, aerospace, energy exploration, automotive, fiber optics and more allowing you to leap past “can’t” and into “how”.
What makes them different is that they think differently. Even though they’re the world’s leading expert in polymer tubing, they’re much more than a polymer tubing company. Zeus solves problems and anticipates innovation. Zeus are in the business of changing lives.
History of Zeus Inc
Frank P. Tourville, Sr. worked in the polymer extrusion industry for ten years before creating Zeus from a desire to “do it better.” While Zeus quickly established itself as a world leader providing high-performance polymer extrusions and solutions, their rapid, sustained growth is rooted in a commitment to improving industries and lives.
Zeus is Everywhere You Are
Zeus touches just about every major industry in the world. Their products make everyday life better, faster, safer. You’ll find Zeus technology in your backyard grill and automobile. You’ll find them in the aircraft taking you where you need to go and the hand of the surgeon saving your life. Zeus' research facilities are surpassed only by the enthusiasm and commitment of our people.
Why Choose Zeus As An Innovation Partner?
When your product and reputation are on the line, you want the best. No other company in the world delivers the consistency, dependability and unrivaled innovation of Zeus.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.