Your first consideration when designing your laboratory workflows and standard operating procedures (SOPs) to follow good laboratory practice (GLP) may not be which brand of pipette to use. However, choosing the right pipette can make achieving consistent results considerably easier.
Each INTEGRA electronic pipette is designed to accommodate the requirements of a modern laboratory environment. They are full of user-orientated features designed to streamline every day pipetting activities and make working to GLP requirements easier.
The VIAFLO and VOYAGER ranges include both fixed and adjustable tip spacing pipettes, ranging from one to sixteen channels. As an alternative, the VIAFLO 96/384 offers a compact, handheld 96- or 384- channel solution.
The INTEGRA electronic pipettes and GLP work hand in hand, let’s take a look.
Calibration

When considering how pipettes are used in a lab, calibration is an important consideration. To ensure accuracy it is essential that pipettes are regularly calibrated but this can be difficult to keep track of when you have a large number of individual pipettes.
INTEGRA has built-in calibration alarms that provide a helpful audiovisual reminder after a period of time and/or number of cycles as defined by the user. This makes life easier. When calibration is required, INTEGRA electronic pipettes can automatically calculate the calibration factor adjustments required. All that needs to be done is to input the results of your gravimetric calibration checks.
Making Routine Tasks Simpler

Another key aspect of GLP is consistency: ensuring that the same protocol is followed by every user, every time. Although SOPs should define how each protocol is carried out, they aren’t always followed rigorously. Even when they are, sensitive workflows or assays may still suffer from variability between operators.
INTEGRA electronic pipettes help to ensure accuracy and consistent performance of dispensing, dilution and mixing steps by offering a range of pre-programmed standard functions such as repeat dispensing and serial dilutions.
They also offer the additional ability to create custom, step-based programs. This can either be done directly on the pipette or using the computer-based VIALINK software, allowing you to build your SOPs directly into the pipette.
Furthermore, you can also incorporate timers, mixing and personalized messages (for example, adding diluents, changing liquids and so on) into the program. This minimizes the risk of errors by helping every user to follow the protocol step-by-step and allows for more consistent assay performance.
Easy Management
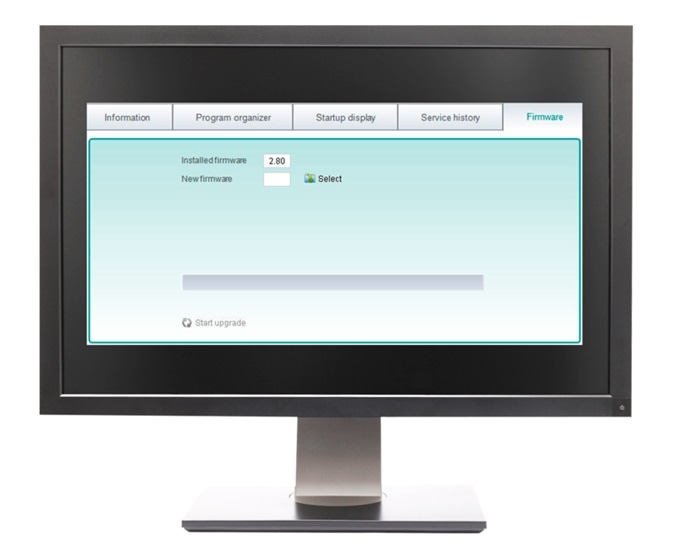
The VIALINK pipette management software makes managing your programs easy. It enables you to name, organize and store each of your custom protocols on a standalone computer or networked drive. This facilitates the creation of a library of programs which serve as back-up. These can also be used to load multiple pipettes with the same program which helps to ensure consistency.
A complete service history for each pipette, including details of calibration factor changes, firmware updates or other alterations to the general pipette settings, is also stored on the software.
If several INTEGRA pipettes are purchased at different times, the software can be used to ensure that all pipettes are upgraded with the latest firmware available.
Password Protection

Process security is important for GLP in many laboratories as it gives users and laboratory managers total confidence in results. INTEGRA allows you to password protect various aspects of pipette firmware including calibration settings, general settings, and standard and custom settings. You can decide which settings can be modified by any user and which to password protect.
For example, you may want to fix your SOPs as custom programs and only allow the standard programs to be changed. Alternatively, you may wish to lock the general and calibration pipette settings – date and time, name of device, sounds, display, language, pipetting speed table – whilst allowing users to modify the pipetting programs. INTEGRA gives you the freedom to make the choice yourself.
About INTEGRA
 INTEGRA provides innovative solutions for Liquid Handling and Media Preparation applications which serve the needs of their customers in research, diagnostics and quality control laboratories.
INTEGRA provides innovative solutions for Liquid Handling and Media Preparation applications which serve the needs of their customers in research, diagnostics and quality control laboratories.
Their instruments and plastic consumables are developed and manufactured in Zizers, Switzerland and Hudson, NH USA. In order to remain close to their customers, they maintain a direct sales and support organization in North America, the UK, France and Germany, as well as a network of over 100 highly trained distribution partners worldwide.
In recent years they have focused on developing a new and technologically advanced range of handheld electronic pipettes which are simple to use and meet the ergonomic needs of their customers.
Today they are proud to offer the widest range of electronic pipettes in the market spanning a range from single channel pipettes up to 384 channel bench-top instruments.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.