In the last 10 years, immune checkpoint blockade has come to light as a viable therapeutic tool against tumors of several types. Checkpoint blockade therapies leverage the fact that immune cells infiltrate various tumors but are unable to destroy the tumor effectively because of inhibitory signals that restrict their function.
Checkpoint blockade therapy reverses the inhibitory signals blocking T cell functionality, thus reactivating immune cells already present in a tumor and enabling them to resume their effector function.1
The PD-1/PD-L1 checkpoint
The PD-1/PD-L1 pathway is the most well-known checkpoint that can be therapeutically targeted. FDA approval for many drugs that target this pathway was, in fact, granted in 2017.2 PD-1, which is a receptor existing on T cells, is upregulated on 'exhausted' T cells that experience chronic antigen stimulation.3
Tumors are rich in antigen, which often make tumor-infiltrating T cells (TIL) quickly start upregulating PD-1. PD-L1 is expressed by various tumor cells on their cell surface.4,5 As a result of the interaction between PD-L1 and PD-1 expressed on T cells, a regulatory signal is transmitted to the T cells.6
Thus, when the interaction between PD-L1 and PD-1 is inhibited, the effector function of TIL is increased, resulting in an anti-tumor immune response that prolongs progression-free and overall survival in a subset of cancer patients.7
The success of anti-PD-(L)1 therapy has led to the testing of many emerging checkpoint inhibitors in model systems. Signaling through these pathways presents an inhibitory effect on T cells, similar to that downstream of PD-1.
BTLA, LAG-3, TIGIT and TIM-3 are some of the receptors used by T cells to downregulate their response to antigen. These receptors have been put forward as innovative therapeutic targets, and many latest reviews emphasize the capability of these novel checkpoint targets.8,9
The ligands to these receptors occur - quite similar to PD-L1 - on the surface of antigen-presenting cells and/or tumor cells, implying that TIL inhibition through these signaling pathways is predicted to take place inside tumors. Researchers have already filed patent applications across the world for therapeutically targeting these receptors.9
One main reason for the constant development of innovative checkpoint blockade immunotherapies is that only some patients respond to CTLA4 or PD-(L)1 blockade, and patients who initially present with a response can later develop resistance to these therapies.10,11
For instance, certain patients present reduced expression of particular checkpoint markers in a biologic escape mechanism to these therapies. However, other molecules with analogous cellular functions could still be targeted to realize similar effects.12
With a new wave of immunotherapies waiting to reach the market, it is a great time to observe the growing prospects of targeting numerous components of the immune system for cancer treatment.
Comparison data
A comparison of Bethyl’s new PD-L1 recombinant rabbit monoclonal antibody with the antibody of a leading competitor showed that Bethyl’s antibody was eight times more sensitive. Antibodies were tested according to the protocol from each manufacturer as given on the product datasheet, which looks to improve antibody performance.
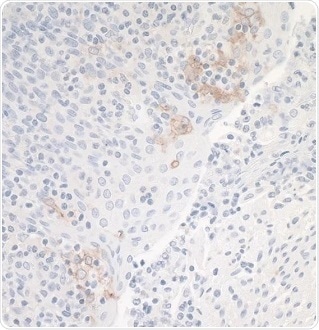
Figure 2. Leading Competitor’s Rabbit anti-PD-L1 Monoclonal Antibody. Application: IHC. Sample: Tonsil. Image Credit: Bethyl Laboratories Inc.
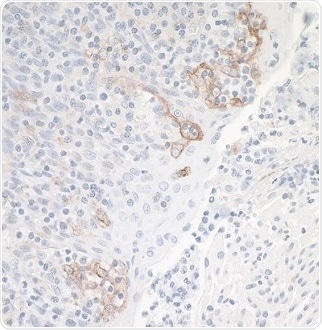
Figure 3. Bethyl’s Rabbit anti-PD-L1 Recombinant Monoclonal Antibody [BLR020E] (A700-020). Application: IHC. Sample: Tonsil. Image Credit: Bethyl Laboratories Inc.
Recommended secondaries
- DyLight® 488-conjugated goat anti-mouse IgG (A90-116D2)
- DyLight® 488-conjugated goat anti-rabbit IgG (A120-201D2)
- DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4)
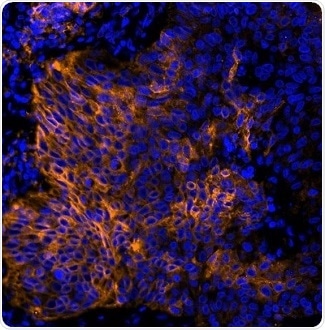
Figure 4. Detection of human PD-L1 (orange) in FFPE lung carcinoma by IHC-IF (pseudo color). Antibody: Rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4). Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
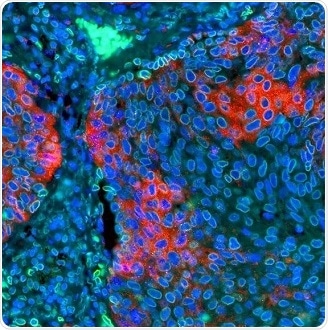
Figure 5. Detection of human PD-L1 (red) and Lamin-A/C (green) in FFPE lung by IHC-IF. Antibody: Rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020) and rabbit anti-Lamin-A/C (A303-430A). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4) and DyLight® 488-conjugated goat anti-rabbit IgG (A120-201D2). Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
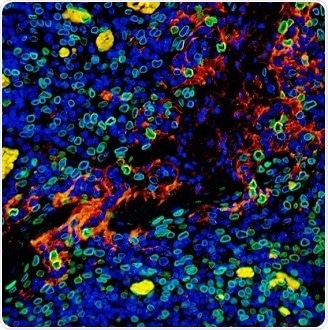
Figure 6. Detection of human PD-L1 (red) and Lamin-A/C (green) in FFPE tonsil by IHC-IF. Antibody: Rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020) and rabbit anti-Lamin-A/C (A303-430A). Secondary: DyLight® 594-conjugated goat anti-rabbit IgG (A120-201D4) and DyLight® 488-conjugated goat anti-rabbit IgG (A120-201D2). Counterstain: DAPI (blue). Image Credit: Bethyl Laboratories Inc.
References
- Zhou G, Sprengers D, Boor PPC, et al. 2017. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. Oct;153(4):1107–1119.e10. doi:10.1053/j.gastro.2017.06.017.
- Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. 2018. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer. Jan 23;6(1):8. doi:10.1186/s40425-018-0316-z.
- Blackburn SD, Shin H, Freeman GJ, Wherry EJ. 2008. Selective expansion of a subset of exhausted CD8 T cells by PD-L1 blockade. Proc Natl Acad Sci. Sept 30;105(39):15016–15021.
- Badalamenti G, Fanale D, Incorvaia L, et al. 2018. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. Jan 30. doi:10.1016/j.cellimm.2018.01.013.
- Gatalica Z, Snyder C, Maney T, et al. 2014. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. Dec;23(12):2965–2970.
- Jin H-T, Ahmed R, Okazaki T. 2010. Role of PD-1 in Regulating T-Cell Immunity. In: Vol Springer, Berlin, Heidelberg:17–37.
- Dong Y, Sun Q, Zhang X. 2017. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. Jan 10;8(2):2171–2186.
- Torphy RJ, Schulick RD, Zhu Y. 2017. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int J Mol Sci. Dec 6;18(12). doi:10.3390/ijms18122642.
- Collin M. 2016. Immune checkpoint inhibitors: a patent review (2010-2015). Expert Opin Ther Pat. May;26(5):555–564.
- Jenkins RW, Barbie DA, Flaherty KT. 2018. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. Jan;118(1):9–16.
- Bai J, Gao Z, Li X, Dong L, Han W, Nie J. 2017. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. Nov 25;8(66):110693–110707.
- Nirschl CJ, Drake CG. 2013. Molecular pathways: coexpression of immune checkpoint molecules: signaling pathways and implications for cancer immunotherapy. Clin Cancer Res. Sept 15;19(18):4917–4924.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.