The field of cancer immunotherapy has witnessed rapid developments, and this trend can be attributed to a better understanding of the presentation of antigens, the expression of immune markers and the cell-regulated immune response involving CD3+ T-lymphocytes.
T-cells are vital to the body’s immune system to mount a response against cancer, both endogenously and after therapeutic intervention. A panel of T-cell markers that can detect subsets of tumor-related T-cells enables researchers to recognize and target subpopulations of T-cells that exclusively contribute to the immune reaction against a tumor.
CD4+ and CD8+ T-cells take part in the anti-tumor immune response either in the periphery, for example, in a tumor-draining lymph node, or at the tumor site. Among the T-cells that are localized to the tumor, a few T-cells penetrate the tumor in the form of naive cells and start to mature inside the tumor.1
Other T-cells are primed in the periphery and then cluster at the tumor site to take part in the memory or effector response against a proven tumor.2 Adhesion molecules, such as CD31,3 CD434 and CD445 help control the relocation of these T-cells.
Inside the tumor microenvironment, a positive prognosis is generally conferred to patients suffering from solid tumors with high infiltration of CD8+ T-cells,6 since cytotoxic T-cells that infiltrate tumors are the critical mediator of the body’s immune response to tumors.
But the activity of CD8+ T-cells can be controlled inside the tumor microenvironment. The degree of both maturation and stimulation of a CD8+ T-cell can be modulated by tumor cells, CD4+ helper T-cells and myeloid cells.
Among the various challenges, T-cell exhaustion and energy are the major ones when it comes to maintaining the anti-tumor immune responses,7 and can be tracked through the variations to the cell surface molecules.
Simultaneously, CD4+ T-cells are capable of supporting an ongoing immune reaction against solid tumors and they achieve this by promoting a Th1-skewed microenvironment that primes the CD8+ T-cells.
Recently, it has been demonstrated in mouse models that Th17-polarized CD4+ T-cells also support anti-tumor immune responses, perhaps because of their memory-like phenotype. But based on the phenotype of the T-cells and the type of cancer, CD4+ T-cells may either confer a negative or positive prognosis.
While the inflammatory response is boosted by helper and memory CD4+ T-cells, ongoing immune responses are suppressed by regulatory T-cells, which can also support the progression of tumors.8
Studies on T-cell markers are important to figure out how the anti-tumor immune response is influenced.
The ability to classify the subsets of T-cells - according to their physiologic localization and maturation condition in various types of human cancers and associated mouse models - will enhance the potential to focus on critical subsets of T-cells for inhibition or activation at different stages of the progression of tumors.
Furthermore, defining molecules that may work as new immune checkpoints or as exhaustion markers within the scope of cancer, such as CD8 and CD5,9 can build on the achievements of anti-PD-1 and anti-CTLA4 treatments, transforming this area into the clinical settings yet again.
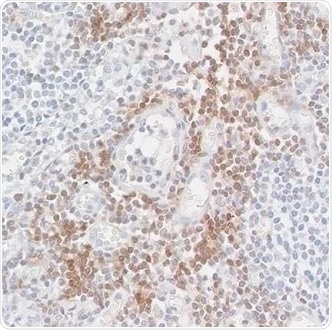
Figure 1. Detection of CD247/CD3Z in an FFPE section of a metastatic lymph node from lung cancer origin by IHC. Antibody: Rabbit anti-CD247/CD3Z recombinant monoclonal antibody [BL-336-1B2], Cat# A700-017. Detection: DAB. Image Credit: Bethyl Laboratories Inc.
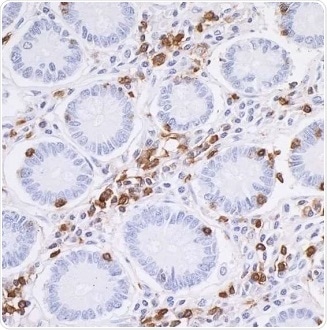
Figure 2. Detection of CD45 in an FFPE section of human small intestine by IHC. Antibody: Rabbit anti-CD45 recombinant monoclonal antibody [BL-178-12C7], Cat# A700-012. Detection: DAB. Image Credit: Bethyl Laboratories Inc.
The current range of T-cell markers from Bethyl Laboratories contains these monoclonal and polyclonal products - including the company’s latest recombinant rabbit monoclonal antibodies (RmAbs).
T-Cell regulation/immune checkpoints
Recommended secondaries
- Rabbit IgG-heavy and light chain cross-adsorbed Antibody (A120-201D3)
- Rabbit IgG-heavy and light chain cross-adsorbed Antibody (A120-201D4)
- Rabbit IgG-heavy and light chain Antibody (A120-101P)
- Rabbit IgG-heavy and light chain highly cross-adsorbed Antibody (A120-501P)
References
- Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. 2016. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 7, 407.
- Slaney CY, Kershaw MH, Darcy PK. 2014. Trafficking of T Cells into Tumors. Cancer Res. 74(24). 7168-7174.
- Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. 2013. An immunologist’s guide to CD31 function in T-cells. J Cell Sci 126, 2343-2352.
- Mody PD, Cannon JL, Bandukwala HS, Blaine KM, Schilling AB, Swier K, Sperling AI. 2007. Signaling through CD43 regulates CD4 T-cell trafficking. Blood. 110(8), 2974-2982.
- Baaten BJ, Li CR, Bradley LM. 2010. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 3(6), 508-512.
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12, 298-306.
- Wherry EJ, Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15, 485-499.
- Kim HJ, Cantor H. 2014. CD4 T-cell Subsets and Tumor Immunity: The Helpful and the Not-so-Helpful. Cancer Immunol Res. Feb;2(2):91-8.
- Tabbekh M, Mokrani-Hammani M, Bismuth G, Mami-Chouaib F. 2013. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2(1), e22841.
About Bethyl Laboratories, Inc.

Bethyl Laboratories, Inc. has been dedicated to improving lives by supporting scientific discovery through its qualified antibody products and custom polyclonal services since its founding in 1972. Bethyl has a global reputation for quality, consistency and first-class customer care. Every antibody that Bethyl sells is manufactured to exacting standards in Montgomery, Texas, and is validated in-house by a team of scientists. From the veterinary facilities to the development, production, and validation labs, the entire Bethyl team focuses on delivering quality products and delighting customers.
Bethyl Laboratories has been acquired by Fortis Life Sciences. To learn more visit: https://promotions.bethyl.com/news/fortis-life-sciences-acquires-bethyl-laboratories/.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.