Antibody-derived biologics have become a major class of modern medicine, particularly in the fight against cancer and autoimmune diseases.
Highly efficacious immunoglobulin-based drugs have been naturally developed by antibody-producing B cells from within the mammalian immune system. Despite this, finding rare cells with the right characteristics remains a challenge.
Mouse B cells have traditionally been immortalized through hybridoma fusion. However, there are numerous problems because B cells die quickly in culture, and many do not survive the fusion process.
As a result, the rare B cell of interest may be lost throughout the hybridoma formation process. The development of hybridoma fusions is time-consuming, costly, and inefficient, as it may not result in the identification and immortalization of the rare antigen-specific antibody secreting B cells of interest.
To screen and isolate rare antigen-specific producing cells, semi-automated technologies like cell sorting, colony picking, and cell-in-well imagers are now employed in tandem.
The Cyto-Mine® Single Cell Analysis and Monoclonality Assurance System is the first completely integrated biopharma platform that inspects millions of primary B cells or hybridomas for secreted immunoglobulin, recognizes and sorts antigen-specific candidates, and gently dispenses them into 96- or 384-well microtiter plates with visual proof of cell number via high-quality imaging (Figure 1).
The current research demonstrates how Cyto-Mine® uses an antigen-specific test to analyze a heterogeneous B cell or hybridoma population in order to recognize target-specific variations in a high-throughput way.
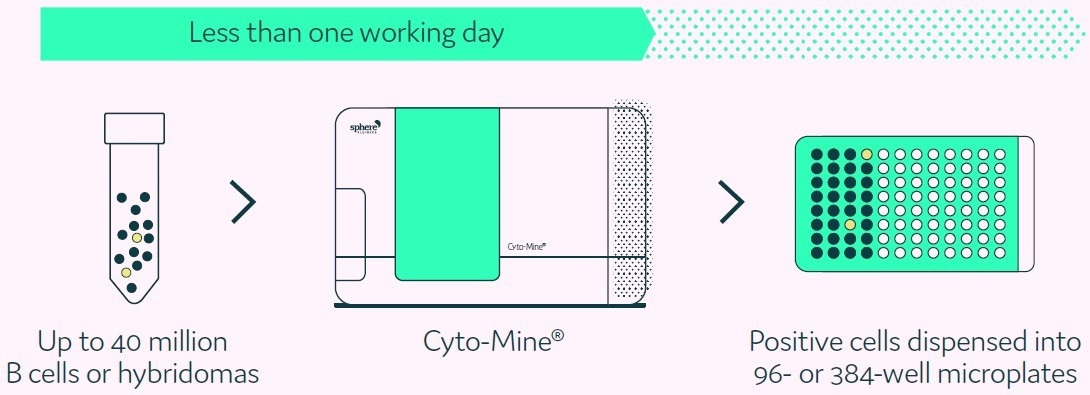
Figure 1. Cyto-Mine® technology finds and isolates cells secreting antigen-specific antibodies from complex cell populations. Image Credit: Fluidic Sciences and Sphere Bio
Aims and objectives
This article will outline how Cyto-Mine® can:
- Precisely identify and isolate “hit” cells from a large hybridoma population using an antigen-specific assay
- Evaluate different concentrations of antigen-specific antibodies
- Use a single completely integrated instrument to substantially simplify the Antibody Discovery workflow
Methods
Single cell encapsulation
Cells from a hybridoma population were diluted to a concentration that maximizes the number of picodroplets containing only a single cell using Poisson distribution statistics. A dilution can be made for exceptionally large populations to allow for the encapsulation of pools of cells in a single picodroplet (Figure 2).
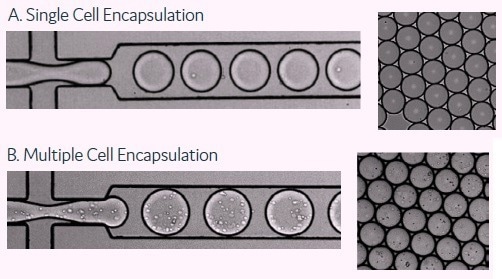
Figure 2. These images show the encapsulation of single cells or multiple cells per picodroplet. A) Cells were diluted to a concentration of 1 × 106 cells/mL to obtain 1 cell per picodroplet. B) A large population of cells (greater than 1 million) were diluted to a concentration of 1 × 108 cells/mL in medium resulting in multiple cells per picodroplet. Image Credit: Fluidic Sciences and Sphere Bio
The culture medium was added with OptiPrep™ Density Gradient Medium (Sigma Aldrich Merck) and a set of detection probes, a fluorescently conjugated antigen donor probe and an immunoglobulin-specific acceptor probe, prior to cell suspension (see Box 1).
After that, the diluted mixture was pipetted into a Cyto-Cartridge® and loaded into Cyto-Mine®. The method begins with Cyto-Mine® encapsulating the cells in 300 pL picodroplets.
The number of cells per picodroplet can be adjusted to meet the needs of the user, for example, one cell per picodroplet if single-cell cloning is required, or more than one cell per picodroplet if the population size exceeds 1 million cells, with the latter resulting in the collection of enriched mini-pools of cells rather than single cells (Figure 2).
The cells were incubated in situ in Antigen-Specific Secretion Assay Next Cyto-Mine® so that the secreted immunoglobulins concentrated inside the picodroplets and could be identified by the antigen-specific detection reagent present in the medium (Box 1).
The miniaturized format provides an ultrasensitive, quick assay (usually with an incubation duration of 0.5 to 2 hours) depending on the cell population’s production rate.
Sorting
The fluorescent antigen-positive hits are sorted according to their fluorescence intensity. Figure 3 depicts the integrated steps of the Cyto-Mine® antigen-specific assay process.
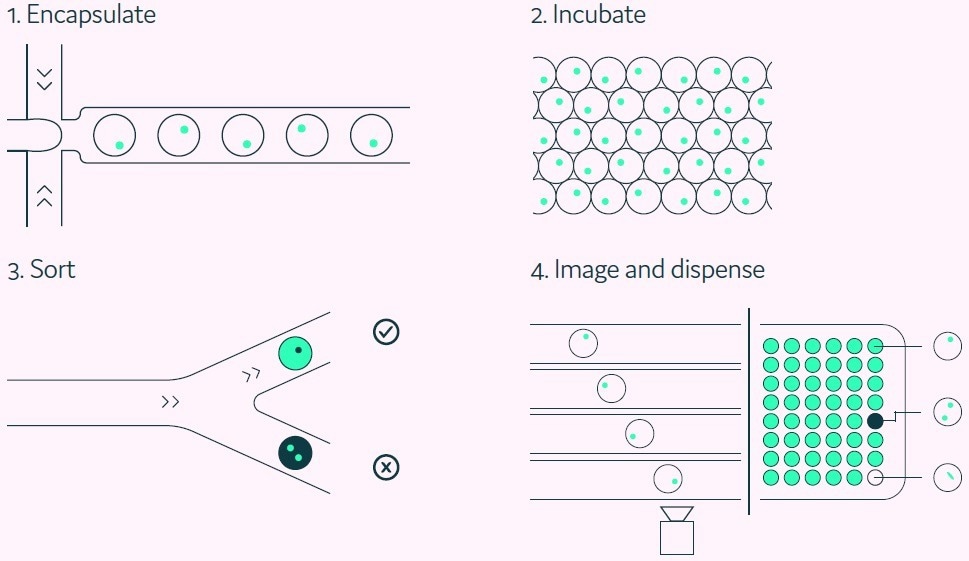
Figure 3. The Cyto-Mine® workflow integrates the screening, sorting, isolation, and verification of antigen-specific clones into a fully automated process. Image Credit: Fluidic Sciences and Sphere Bio
Clonality assurance
Prior to dispensing, as the picodroplet travels through the final dispensing microfluidic channel, the encapsulated cells are imaged multiple times to provide verification of clonality.
Box 1. Antigen-specific secretion assay
A key enabling component of Cyto-Mine® is its ability to analyze the secreted immunoglobulins of millions of cells for antigen specificity while maintaining the cells in a highly viable state with no prior modification.
Figure 4 depicts the procedure for reporting positives. The detection probe pair (fluorescently conjugated antigen and an immunoglobulin-specific acceptor probe) binds to the released antigen-specific immunoglobulin, causing a fluorescence shift via FRET.
The fluorescent signal created is subsequently measured and converted into a quantitative output by Cyto-Mine®. The use of an acceptor probe allows for the simultaneous detection of antigen specificity and immunoglobulin isotype.
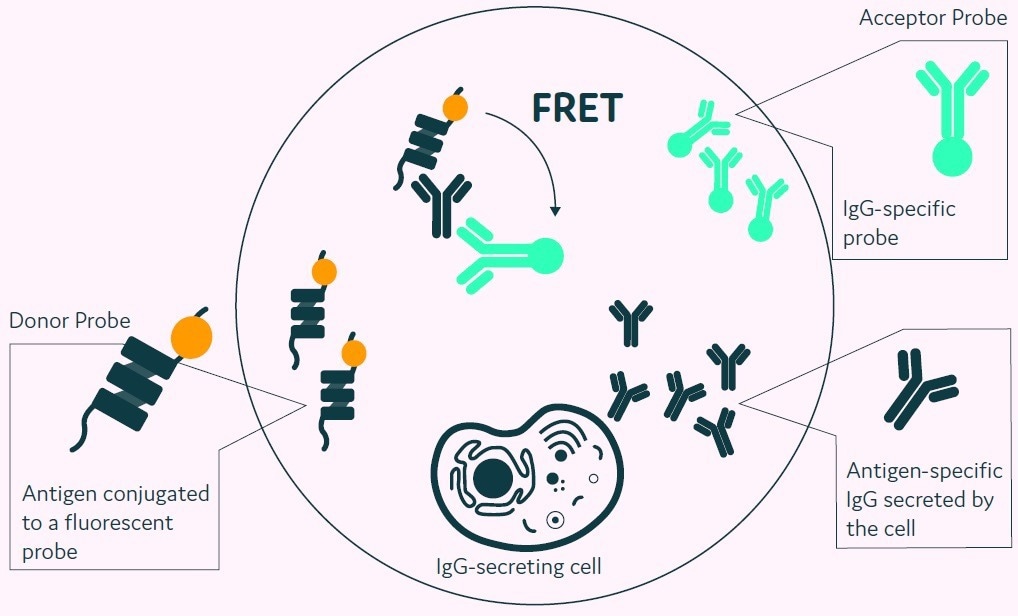
Figure 4. The Cyto-Mine® picodroplet-based antigen-specific assay. This model shows the standard assay to screen for antigen-specific IgG. Antigen-specific IgG secreted from the encapsulated cell during incubation is recognized by both the antigen and the IgG-specific acceptor probe forming a 3-body FRET complex that induces a fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
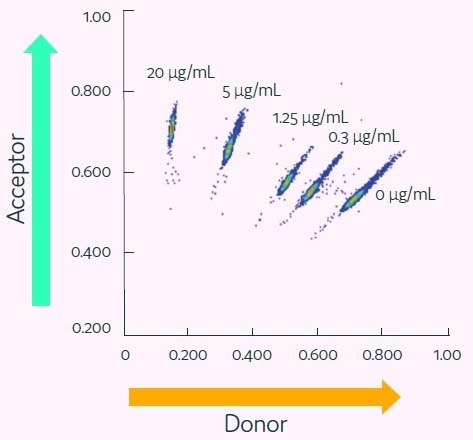
Figure 5. Cyto-Mine® Scatter Plot. Large numbers of individual picodroplets were loaded with the indicated concentrations of anti-human TNF-alpha IgG and then resolved using the Cyto-Mine® antigen-specific assay and analysis. Image Credit: Fluidic Sciences and Sphere Bio
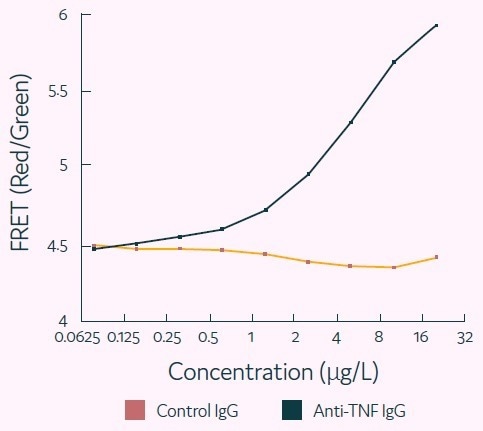
Figure 6. Representative titration curve generated using the Cyto-Mine® antigen-specific assay. In this example, the control IgG confirms specificity of the assay for human TNF-alpha. Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.