Cell line development (CLD) follows a process that has only changed slightly since it was established in the early days of biopharmaceutical development.
The steps to create a stable, manufacturing cell line include host cell line selection, engineering of the expression vector, transfection, cloning and cell expansion, as well as various screening steps to select the clone with the highest cell viability, growth, expression level, stability and product quality.
However, there are a variety of ways to help companies enhance their workflow within this process.
Dr. Jon Dempsey offers his opinions on the best methods for enhancing CLD procedures in this article.
Jon Dempsey is the founder and CEO of Pathway Biopharma. He has worked in the biopharmaceutical sector for close to 30 years, bringing expertise in biologics chemistry, manufacture and control (CMC) including cell line development, cell therapy and process development, analytics and protein chemistry, CDMO selection and manufacturing.
Over this period, Dempsey has participated in the development of numerous commercial biotherapeutics.
Dempsey was asked for his advice, for both seasoned researchers and scientists as well as those just starting out, regarding the best ways to advance in CLD.
Insights from Jon Dempsey
As CLD and the development of the first clinical batch is on the critical path for advancing a novel therapeutic to a clinical trial, companies are looking for any ways to speed up the development process.
These four steps Dempsey would take if he was building a CLD system:
- Create the workflow, then, using directed evolution or clonal selection, develop the cell line to meet this workflow
- Utilize artificial vector technology to enhance expression
- Utilize automation, such as Cyto-Mine®, to eliminate manual processes and accelerate the procedure by preventing rounds of single-cell cloning
- Reduce the duration between cell doublings to encourage rapid cell growth
Design the cell line to fit any workflow
Chinese Hamster Ovary (CHO) cell lines
CHO cells almost exclusively generate antibodies for therapeutic application. This is because CHO cells represent a recognized regulatory system that has received FDA approval.
Most biopharmaceutical businesses adhere to this regulatory pathway as it offers a tried-and-true road to market, as CHO cells were primarily used to manufacture the first authorized recombinant biopharmaceuticals. As such, CHO cells are used to produce >70% of all recombinant proteins.
Using CHO cells has a lot of scientific advantages in addition to being a regulatory method that has been approved by the FDA. They can grow in suspension culture and in chemically defined, serum-free, animal-origin-free medium, which allows for increased performance across various cultures and large-scale manufacturing.
High levels of gene expression are frequently seen in CHO cells, which increases protein production and specific productivity.
Expression systems based on CHO cells could be more resilient and adaptable to alterations in the culture environment. Additionally, and probably most crucially, CHO cells offer a “human-like” glycosylation profile that has the potential to stop a patient’s immune system from being activated, which could result in undesirable side effects or a loss of product efficacy.
Despite the preference for CHO cells, the industry does not employ a single, well-defined cell line or even a typical CHO host cell line. The majority of companies will use a parental line obtained from a cell culture collection as the basis for their own CHO host cell line.
Cells will be kept in a cell bank after being modified with serum and suspension according to the company’s method. In the end, this causes a divergence in cell phenotypes and variations in cell function.
New arrivals to the sector tend to be more cautious when selecting a cell line to employ because CHO cells are so extensively used. Since these technologies are still in their infancy, several companies have attempted to produce products using less conventional cell lines, such as Per. C6® or other human cells, but have been unsuccessful.
The key goals when choosing which cell lines to employ are to make sure that the final clone can colonize from a single cell, generate large yields, and offer good product quality.
To enhance cell outgrowth during single-cell cloning, the majority of companies have modified their cell line and culture medium. In certain CLD approaches, companies begin by employing directed evolution to optimize their cell line to find a cell line that works well for their workflow.
Directed evolution
Typically, during the development of cell lines, multiple screening stages that assess cell characteristics, such as growth and productivity, are used to manage cell line heterogeneity and reduce the number of cell lines that progress onto further assessment stages.
The CLD process can be improved by using directed evolution, which offers a far more effective method of producing enhanced mammalian cell hosts.
Directed evolution provides a method to control the heterogeneity of cells during CLD right from the start by pre-adapting the cells to the development process, as opposed to the conventional method of taking a cell line through the development stages and making decisions throughout the process as would be done traditionally.
For instance, in directed evolution, the cell line could be enriched and sorted to identify subclones that have desired properties for manufacturing, and these subclones could then be used as the host parental cell line to ensure that they can proliferate throughout the development process (Figure 1).
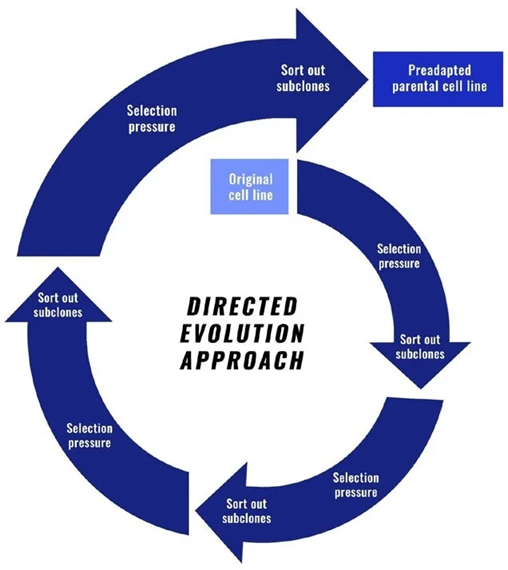
Figure 1. Representation of the directed evolution process. Image Credit: Fluidic Sciences and Sphere Bio
Leverage synthetic promoter technologies
To create a recombinant cell line, customized expression vectors containing the desired product (therapeutic protein) are transfected into the host cell. To allow more successful clone selection, these expression vectors can be built to incorporate elements that raise protein expression levels.
In essence, the expression vector is a piece of DNA that contains elements like the antibody gene, selection marker genes, and genes that permit expression in CHO cells. To speed up the expression of the genetic vector in the host cell line, the selection marker gene’s main function is to promote it. Glutamate synthetase (GS) and dihydrofolate reductase (DHFR) are two conventional selection indicators for CHO cells.
The selection marker gene is often controlled by a promoter, which is most frequently endogenous or viral. For industrial-scale operations, viral and endogenous promoters can, however, offer functionality that is unexpected and unmanageable. This opens up the possibility of enhancing protein expression through the investigation of synthetic promoters.
Promoters and other genetic components for CHO cell engineering are being developed by several companies and academic institutions. It is possible to increase expression by matching the promoter sequence to the host cell’s transcriptional machinery.
Synthetic promoters behave consistently and offer a lot more transcriptional control than viral promoters, facilitating advancements in the production of biopharmaceuticals.
For instance, users can create a CHO promoter that would function differently in a gene therapy vaccination. Each promoter would only be able to express itself under particular circumstances or in particular tissues, for example.
Image Credit: Fluidic Sciences and Sphere Bio

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.