The use of high-throughput technologies for single B cell screening offers numerous advantages over traditional hybridoma technology in the discovery of lead therapeutic antibodies.
Hybridoma technology, introduced by Köhler and Milstein in 1975, has been highly successful in producing FDA-approved therapeutic antibodies.1 However, a major drawback of this technology is the limited number of hit candidates within a large cell population.
Only a small portion of the total B cell population from an immunized animal is selected for the fusion reaction, with only a tiny fraction secreting antibodies. The number of cells secreting antibodies specific to the target of interest is even smaller.
During cell processing and fusion, many of these key antibody-secreting cells are lost, potentially leading to the loss of valuable and potent hits. The use of technology that can screen the entire B cell population is essential to ensure that key target-specific antibody-secreting cells are not missed.
Cyto-Mine® provides a solution to the challenge of identifying rare cells from large B cell populations in the discovery of therapeutic antibodies. The platform offers a pain-free process to advance and expedite antibody discovery.
This article highlights the unique picodroplet technology of Cyto-Mine® that offers a reliable and high-throughput platform for screening the entire B cell repertoire for antigen-specific antibody-secreting cells in just two days while preserving cell viability.
Cyto-Mine® can efficiently screen, select, and dispense antibody-secreting cells with antigen specificity.
The platform is capable of encapsulating up to 40 million cells in a single run, assessing for a target antigen, selecting antigen-specific antibody-secreting cells, and dispensing them as a pooled population or as individual cells into 96- or 384-well microtiter plates.

The Cyto-Mine® Single Cell Analysis System. Image Credit: Fluidic Sciences and Sphere Bio
Aims and objectives
- Demonstrate how to use Cyto-Mine® to robustly scan complete B cell repertoires for antigen-specific antibody-secreting cells.
- Showcase the ability of Cyto-Mine® to rapidly screen multiple cells/picodroplets (40 million cells) or single cells/picodroplet (1-2 million cells) in one day.
- Reveal assay flexibility of Cyto-Mine® (antigen-specific assay or more general IgG assay).
Method
Sample preparation
To prepare the frozen lymphocytes from an immunized mouse for analysis, the cells were first thawed and washed with a fresh culture medium. This was achieved by centrifuging the cells at 200 g for 5 minutes and resuspending the cell pellet in a fresh culture medium at room temperature.
The centrifugation process was repeated and the cell pellet was resuspended in Encapsulation Medium (EM), as indicated in Table 1. The cell suspension was then filtered through a 30 µm cell strainer to eliminate any cell clumps or debris.
The cells were counted and diluted to the desired concentration using Encapsulation Medium. To 1 mL of the cell sample, either IgG or antigen-specific FRET assay reagents were added, and the sample was pipetted into a CytoCartridge® and loaded into the Cyto-Mine®.
Source: Fluidic Sciences and Sphere Bio
| Encapsulation Medium Component |
% (v/v) |
| Preferred culture/cloning medium (fresh) |
74% |
| OptiPrep™ Density Gradient Medium (Iodixanol 60% w/v) 16% |
16% |
| Pluronic™ F-68 (10%) |
10% |
Cyto-Mine® workflow
The Cyto-Mine® platform begins by encapsulating cells within 450 pL picodroplets of the desired culture medium, as illustrated in Figure 1. The number of cells per droplet is optimized by using cells at a suitable dilution level.2
Once the cells have been encapsulated, they are incubated to reach detectable levels of secreted antibodies and assayed to identify the secreted antibodies using a previously established assay.
In this study, both an IgG assay and an antigen-specific assay were used over two days. The Cyto-Mine® platform selectively sorts the "hits" and then during subsequent dispensing into individual microtiter plate wells, images the picodroplets for assurance of clonality.
An initial assay validation step was conducted to guarantee that the FRET assay probes were capable of selectively detecting IgG and antigen-specific antibodies secreted from B cells isolated from mouse lymphocytes.
The screening of 40 million cells for antigen-specific antibody-secreting B cells was conducted using a two-step approach with Cyto-Mine®.
The first round involved analyzing the whole B cell repertoire for the enrichment of picodroplets containing cells secreting IgG antibodies. The second round involved isolating single B cells secreting antigen-specific antibodies.
This study utilized the flexibility of Cyto-Mine® to accommodate both pooling of cells in picodroplets for the first round of screening (Figure 1B) and single-cell encapsulation for the second round (Figure 1A).
The cell concentration in the sample was used to calculate the number of cells encapsulated per picodroplet based on Poisson distribution statistics (Table 1).2

Figure 1. These images show the encapsulation of single cells or multiple cells per picodroplet. A) A large population of cells (greater than 1 million) were diluted to a concentration of 1x108 cells/mL in medium resulting in multiple cells per picodroplet. B) Cells were diluted to a concentration of 1x106 cells/mL to obtain population of mostly 1 cell per occupied picodroplet. Image Credit: Fluidic Sciences and Sphere Bio
Cyto-Mine® assays
Cyto-Mine® can quantify the particular secretion of each cell, which is one of its primary enabling features. Before loading the starting cell population onto Cyto-Mine®, the starting cell population is mixed with the appropriate detection probe pairs.
As seen in Figures 2 and 3, the detection probes attach to the released antibodies of interest, creating a FRET-mediated fluorescent signal.
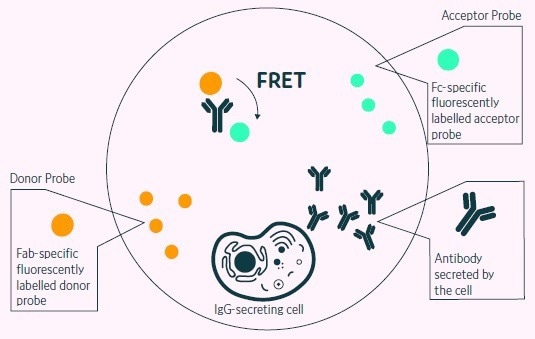
Figure 2. Positive events readout using the Cyto-Mine® picodroplet-based FRET IgG secretion assay. This model shows the standard assay to screen for IgG secreting cells. A customized pair of IgG-specific fluorescent probes are trapped within each picodroplet during encapsulation. IgG secreted from the encapsulated cell is recognized by the detection probe pair forming a 3-body FRET complex that induces a shift in fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
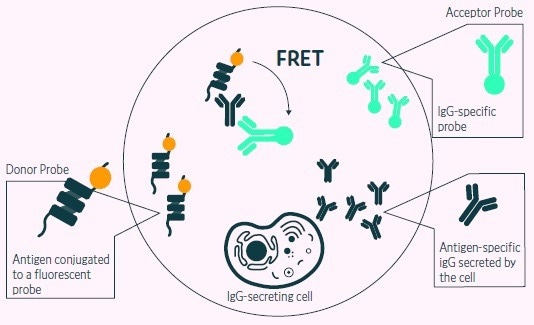
Figure 3. Positive events readout using Cyto-Mine® picodroplet-based FRET antigen-specific assay. A customized pair composed of fluorescent antigen and fluorescent IgG probe are trapped within each picodroplet during encapsulation. Only Antigenspecific IgG secreted from the encapsulated cell is recognized by the detection probe pair forming a 3-body FRET complex that induces a shift in fluorescent signal. Image Credit: Fluidic Sciences and Sphere Bio
Results
Assay validation
A sample of B cells derived from mouse lymph nodes was evaluated using Cyto-Mine®. The analysis consisted of two steps: first, to identify picodroplets containing cells that secrete IgG, and second, to determine if the secreted antibodies could be detected by an antigen-specific probe developed for the target antigen.
The results from these assays are displayed in Figure 4, where it was determined that the isolated B cells secreted either general IgG (left panel) or target antigen-specific antibodies (right panel).
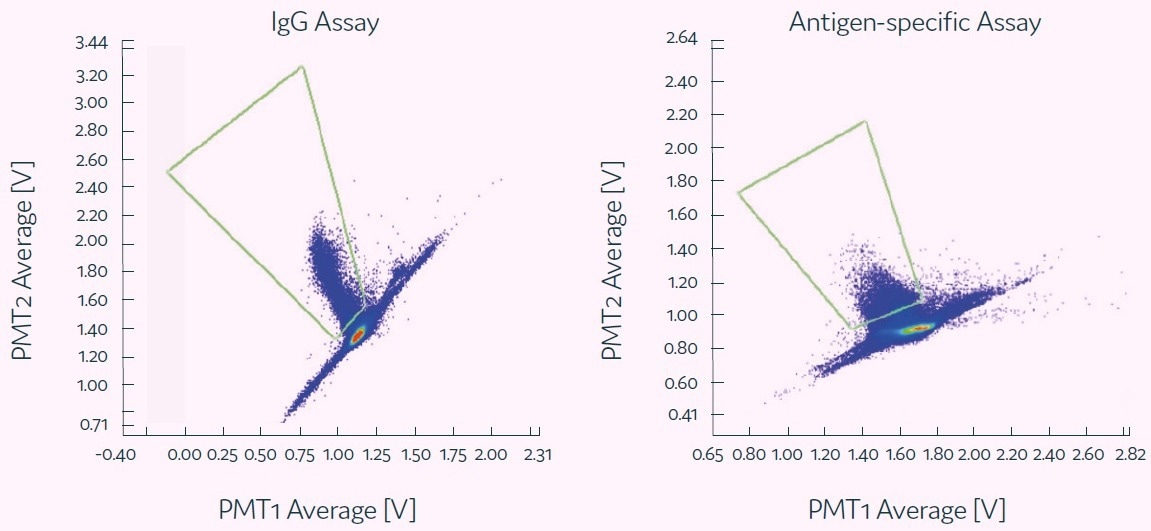
Figure 4. Each of these scatter plots shows representative example of a positive population obtained with different assays indicating the binding of the assay probes to the secreted antibodies. Image Credit: Fluidic Sciences and Sphere Bio
| . |
. |
| Viability of input cell sample |
75.4% |
| No. cells screened |
3.9x107 |
| No. cells per picodroplet (λ) |
32.7 |
| Measured positive rate for current experiment |
0.22% |
| Viability of cells collected from positive hits |
61.20% |
Figure 5. Multiple B cell screening. Image Credit: Fluidic Sciences and Sphere Bio
| Percentage of viable cells |
| Cells viability prior to Cyto-Mine analysis |
75% |
| Cells viability post Cyto-Mine analysis |
61% 61% |
| Cells viability after overnight culture |
65% 65% |
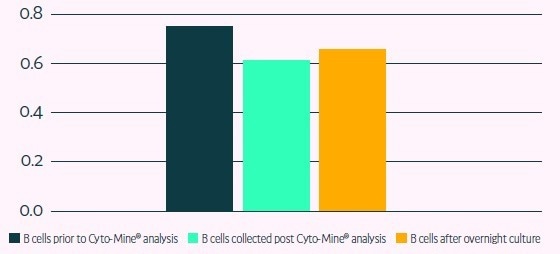
Figure 6. Primary B cell viability after Cyto-Mine® runs Image Credit: Fluidic Sciences and Sphere Bio
| . |
. |
| Viability of input cell sample |
65.4% |
| No. cells screened |
421,300 |
| No. cells per picodroplet (λ) |
0.36 |
| Measured positive rate for current experiment |
0.06% |
Figure 7. Single B cell screening. Image Credit: Fluidic Sciences and Sphere Bio
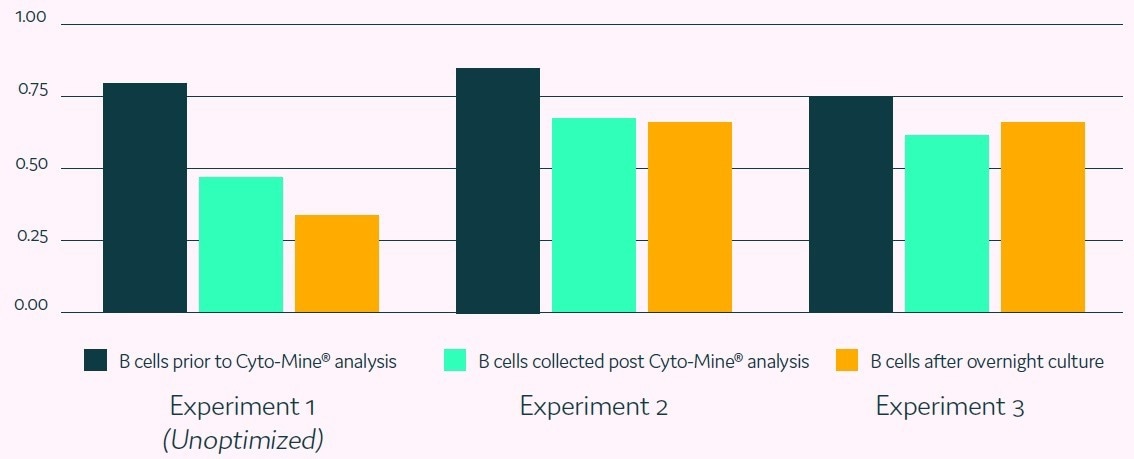
Figure 8. Repeated single B cell screening experiments. Image Credit: Fluidic Sciences and Sphere Bio
References
- Lu, Z.-J.et al., 2012. Frontier of therapeutic antibody discovery. World Journal of Biological Chemistry, pp. 187-196.
- Josephides, D. et al. (2020). Cyto-Mine: An Integrated, Picodroplet System for High-Throughput Single-Cell Analysis, Sorting, Dispensing, and Monoclonality Assurance. SLAS TECHNOLOGY: Translating Life Sciences Innovation. https://doi.org/10.1177/2472630319892571

 Download the full paper
Download the full paper
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.