Human papillomavirus (HPV) is a DNA virus known to cause infections in mucous membranes and skin, typically resulting in warty lesions. While often benign, these lesions do have the potential to become malignant. There are several strains of HPV, some being more likely to cause malignancies, particularly when found in the genital tract.1
There are an estimated 300 million women infected worldwide with HPV. A common sexually transmitted infection, HPV can also be linked to over 311,000 deaths from cervical cancer annually.2 Over 95% of cervical cancer cases are caused by HPV, with only two strains –known as 16 and 18 – responsible for almost 50% of high-grade cervical pre-cancers.3
The HPV vaccine has been deployed in 111 countries, offering hope to eliminate cervical cancer as a global public health problem.2
In late 2022, Türkiye’s Minister of Health, Fahrettin Koca, announced government plans for an HPV vaccination program that aims to meet the WHO target of 90% vaccination rate of the targeted population by 2030.4 The program includes an increase in HPV screening access and the development of a custom HPV diagnostic test.5
The challenge
In Türkiye, a screening project to test for the incidence rate of HPV in 4.6 million women began. AtiTECH and Pharmaline became partners to take on the task of creating and supplying the PCR test kits and all required components.
There are more than 40 HPV strains responsible for lesions in the genital tract. Commercial qPCR kits are available for HPV detection; however, adopting a custom solution enabled the Turkish Government to place its testing resources on the strains endemic to their region. Pharmaline identified that 16 HPV strains represented nearly all the strains in Türkiye. With this knowledge, the team wanted to create a robust, accurate, and specific assay to these select strains in as few reactions as possible per patient sample.
Image Credit: LGC Biosearch Technologies
The solution
Pharmaline could identify the required sequence data to design the test, but support was needed in choosing the correct markers and technology for the probes.
AtiTECH utilized a significant existing relationship with LGC Biosearch Technologies and approached their assay design and development (ADD) team for assistance.

Image Credit: LGC Biosearch Technologies
The ADD team has the expertise needed to provide case-by-case support and to design an assay that can detect the selected high and lower-risk HPV types, and to include internal controls.
After reviewing the sequence data, probes were designed to incorporate the ideal technology for each sequence, and to allow for greater multiplexing per tube.
The team worked with a combination of Minor Groove Binder (MGB) Probes, internal quenching (BHQnova™ Probes) and BHQ™ Probes, as well as a selection of fluorophores.
The final assay was run in only two reactions while detecting all the required HPV targets, including the high-risk types 16, 18 and 45. This was the first time AtiTECH combined different probe technologies in a single kit.
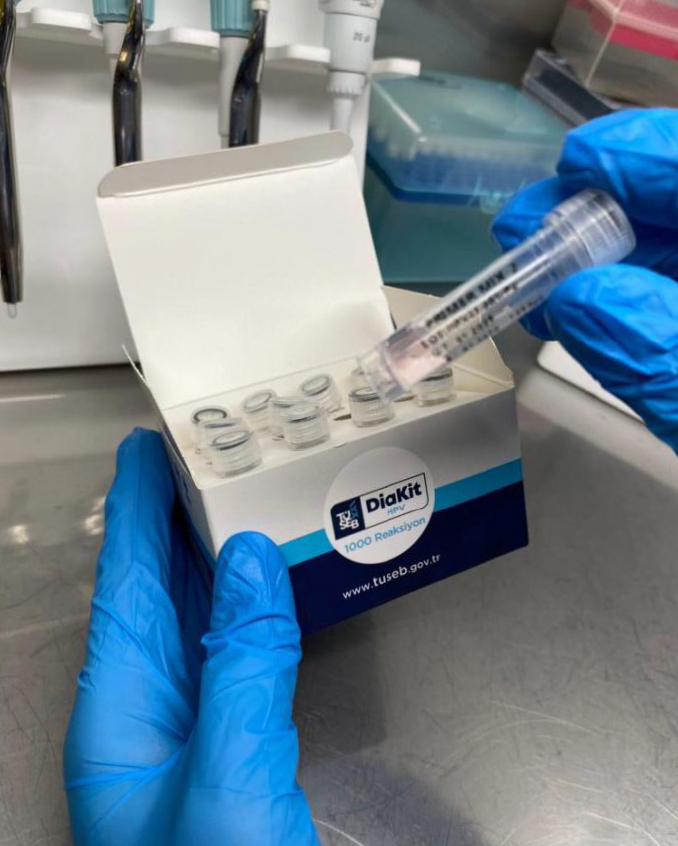
Image Credit: LGC Biosearch Technologies
The result
AtiTECH and Pharmaline opened a lab in Ankara specifically for HPV testing and started to supply the TUSEB Diakit HPV kits. It also completed the testing workflow from DNA isolation through to results. Its client is happy with the results, made possible by the partnership with the assay design and development team at Biosearch Technologies.
The future
The HPV testing project is successfully underway, allowing AtiTECH to look toward the future. Work has begun with the Biosearch Technologies ADD team to create a single-reaction HPV kit capable of identifying 16 strains in a single reaction, as well as a PCR panel for HIV and gastrointestinal diseases.
References and further reading
- Collier L, Oxford J. (2006) Human virology. Third Edition. London Oxford University Press. Pp. 119-126.
- www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis)
- www.who.int/news-room/fact-sheets/detail/cervical-cancer
- https://cancerworld.net/hpv-vaccination-set-to-be-rolled-out-across-turkey-with-controversial-exclusions/
- https://m.bianet.org/english/women/270557-free-hpv-vaccination-will-start-in-turkiye-after-years-long-campaign
About LGC Biosearch Technologies
LGC Biosearch Technologies™ is a global leader in the design, development, and manufacture of sophisticated custom oligonucleotide-based tools and mission-critical components for the molecular diagnostic, research and applied markets. With operations in 18 countries, LGC’s products and services are sold in around 180 countries globally with manufacturing sites across the world to effectively serve you wherever you are located.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.