Introduction
Overview of the ovarian and reproductive tract microbiome
The ovarian microbiome and fertility
Ovarian microbiota and PCOS pathophysiology
Clinical implications and therapeutic potential
Research limitations and future directions
Conclusions
References
Further reading
The ovarian microbiome remains a debated concept, with some studies suggesting microbial influences on fertility and PCOS while others find no consistent microbial signature beyond contamination. Current evidence highlights both potential therapeutic opportunities and critical methodological challenges.
 Image Credit: Shot4Sell / Shutterstock.com
Image Credit: Shot4Sell / Shutterstock.com
Introduction
For over a century, the ‘sterile womb’ dogma considered the upper female reproductive tract (FRT), which comprises the uterus, fallopian tubes, and ovaries, a sterile environment. Modern culture-independent molecular techniques, particularly 16S ribosomal ribonucleic acid (RNA) (16S rRNA) gene sequencing, have provided reports suggesting the existence of distinct microbial communities throughout the upper FRT, but this remains actively debated due to the extreme low biomass of these sites and the high risk of contamination in many study designs.3,4,10
These discoveries have sparked interest in the role of the ovarian microbiome in reproductive function. In fact, some observations suggest that conditions like idiopathic infertility and polycystic ovary syndrome (PCOS) may arise due to dysfunctional host-microbiome systems, which may offer novel approaches to diagnose and treat these painful conditions.1,2
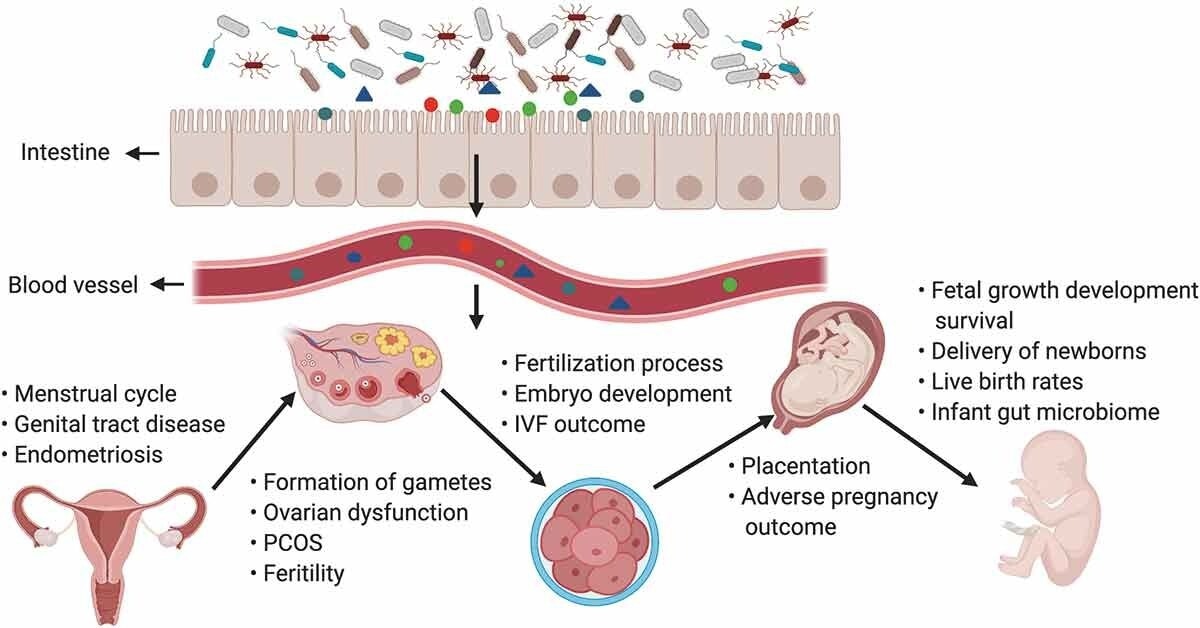
The gut microbiota and its impact on the female reproductive tract, embryo development, and pregnancy. Products of gut microbiota may be transported through the circulation and influence the female reproductive tract (e.g., may cause disruptions in the menstrual cycle, genital tract disease, and endometriosis), ovarian function, embryonic development, and the health of the mother and fetus. The gut can affect the formation of gametes, embryo development, and fertilization processes, and alteration of intestinal flora can lead to ovarian dysfunction, PCOS, infertility, and adverse IVF outcomes. In pregnant women, the gut microbiota has an effect on placentation, pregnancy outcomes, the delivery of newborn babies, the infant microbiome, the live birth rate, and fetal growth, development, and survival.1
Overview of the ovarian and reproductive tract microbiome
Novel sequencing technologies have revealed a ‘microbiota continuum’ along the FRT that is characterized by a proposed gradient of decreasing microbial biomass and increasing microbial diversity from the vagina to the upper FRT.4
Although the vagina is typically dominated by a few Lactobacillus species, the uterus, fallopian tubes, and ovaries have been reported to harbor a more diverse, low-biomass community. For example, follicular fluid and ovarian tissues have been reported to include taxa such as Lactobacillus and Bifidobacterium alongside opportunistic genera such as Streptococcus, Staphylococcus, Gardnerella, and Prevotella. However, high-quality evidence for stable, resident communities in the healthy upper FRT remains limited, and some recent studies suggest any apparent signal may largely reflect contamination in low-biomass samples.4,5,10
Many of these discoveries have been achieved through the use of 16S rRNA sequencing, which identifies bacteria by their genetic material. More recently, high-resolution methods like 2b restriction site associated DNA (2bRAD) sequencing for microbiome (2bRAD-M) have emerged, thereby enabling scientists to better characterize these low-biomass communities, especially since host DNA contamination is a significant challenge for conventional sequencing technologies.6
The ovarian microbiome and fertility
Human follicular fluid (FF) has been well characterized and provides the optimal microenvironment for oocyte development. However, the microbial composition of FF is increasingly implicated as a critical determinant of oocyte quality and in vitro fertilization (IVF) success.5
The presence of Lactobacillus in FF is significantly associated with higher oocyte maturation rates and successful embryo transfer. In contrast, dysbiotic bacteria like Propionibacterium, Streptococcus, and Staphylococcus are frequently associated with lower-quality embryos, reduced transfer rates, and failed implantation, suggesting that the FF microbiome functions as a quality control checkpoint, actively shaping the developmental competence of the oocyte. These links are largely observational (and often culture-based) rather than causal, so mechanistic inferences should be made cautiously.2,5
These alterations arise due to complex interactions between microbial metabolites, local immunity, and hormonal signaling. For example, pro-inflammatory bacteria can induce the release of cytokines within the follicle, which subsequently creates a state of chronic, low-grade inflammation and oxidative stress.
Oocytes are highly sensitive to this oxidative stress, which can compromise their quality by damaging DNA, proteins, and lipids.Microbial enzymes may also alter the concentrations of essential hormones like estrogen and progesterone within the FF, thereby disrupting the endocrine balance required for oocyte maturation.1,7
 Image Credit: Shot4Sell / Shutterstock.com
Image Credit: Shot4Sell / Shutterstock.com
Ovarian microbiota and PCOS pathophysiology
PCOS is the most common endocrine disorder in reproductive-age women that is characterized by hyperandrogenism, ovulatory dysfunction, and metabolic disturbances like insulin resistance. Systematic reviews consistently demonstrate that PCOS patients exhibit reduced species diversity and an enrichment of pro-inflammatory genera such as Bacteroides and Escherichia.7,8
The link between gut dysbiosis and PCOS is explained by the ‘gut-ovary axis,’ a bidirectional communication network with notable physiological associations. During gut dysbiosis, the intestinal barrier becomes more permeable, which allows bacterial components like lipopolysaccharide (LPS) to enter the bloodstream.8
Circulating LPS leads to chronic, low-grade systemic inflammation that stimulates the ovaries to overexpress androgens and simultaneously contributes to insulin resistance in peripheral tissues. The resulting hyperinsulinemia further stimulates ovarian androgen production to create a positive feedback loop.1,7
Overall, current human evidence is predominantly associative, with heterogeneous cohorts and analytic methods; nonetheless, converging data support LPS-driven inflammation and hormone dysregulation as plausible mechanisms linking gut dysbiosis to PCOS features.1,7,8
Clinical implications and therapeutic potential
Supplementation with probiotics, prebiotics, and synbiotics has been shown to significantly improve clinical outcomes in women with PCOS. These supplement regimens reduce insulin resistance and total testosterone levels, while simultaneously increasing sex hormone-binding globulin (SHBG) levels to mitigate hyperandrogenism. However, effects vary by strain, dose, and trial design, and most RCTs are short in duration with notable heterogeneity, so standardized protocols and head-to-head comparisons are still needed.9
Microbiome analysis also has significant potential as a diagnostic tool for profiling microbial communities within both the gastrointestinal (GI) and reproductive tracts. These analyses could help identify dysbiosis as an underlying cause of infertility, particularly in cases of recurrent IVF failure or idiopathic patients who fail to conceive for hitherto unknown reasons.2
Despite these potential benefits, establishing clear causality remains a challenge in human studies. High inter-individual variability in microbiome composition and structure further complicates the definition of a ‘healthy’ baseline profile.9
Your Vaginal Microbiome: The Hidden Key to Fertility
Research limitations and future directions
The primary historical barrier in microbiome research is high signal-to-noise in low-biomass studies. The minute amount of microbial DNA in ovarian tissue or follicular fluid makes samples highly susceptible to contamination, especially from host genetic material.10
Recent low-biomass investigations underscore this concern: for example, one 2025 assessment of ovarian samples reported that most yielded no amplifiable bacterial DNA and that readouts were frequently indistinguishable from reagent/background contaminants, arguing against a robust, distinct ovarian signature.10
To overcome these limitations, longitudinal studies that monitor any changes in the composition of the microbiome over time using next-generation multi-omics approaches are essential. Integrating metagenomics with metatranscriptomics, metaproteomics, and metabolomics data will further clarify the functional impact of these microorganisms.6
Conclusions
The discovery of microbial ecosystems within the upper FRT and subsequent establishment of its importance in reproductive biology has transformed the landscape of reproduction-centric interventions.
Recent advancements suggest that microbiome-targeted therapies like synbiotics have the potential to improve the metabolic and hormonal profiles of women with PCOS. At the same time, the ovarian compartment may not harbor a stable, resident microbiome distinguishable from contamination in many datasets, so there remains an urgent need for larger, longitudinal studies that employ standardized, contamination-controlled methodologies and functional, multi-omics analyses.10
References
- Qi, X., Yun, C., Pang, Y., & Qiao, J. (2021). The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 13(1). DOI:10.1080/19490976.2021.1894070, https://www.tandfonline.com/doi/full/10.1080/19490976.2021.1894070
- Gullo, G., Satullo, M., Billone, V., et al. (2025). The Role of the Genital Tract Microbiome in Human Fertility: A Literature Review. Journal of Clinical Medicine 14(9); 2923. DOI:10.3390/jcm14092923, https://www.mdpi.com/2077-0383/14/9/2923
- Perez-Muñoz, M. E., Arrieta, M., Ramer-Tait, A. E., & Walter, J. (2017). A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: implications for research on the pioneer infant microbiome. Microbiome 5(1). DOI:10.1186/s40168-017-0268-4, https://microbiomejournal.biomedcentral.com/articles/10.1186/s40168-017-0268-4
- Chalif, J., Wang, H., Spakowicz, D., et al. (2024). The microbiome and gynecologic cancer: cellular mechanisms and clinical applications. International Journal of Gynecological Cancer 34(2); 317-327. DOI:10.1136/ijgc-2023-004894, https://www.sciencedirect.com/science/article/pii/S1048891X24012969
- Pelzer, E. S., Allan, J. A., Waterhouse, M. A., et al. (2013). Microorganisms within Human Follicular Fluid: Effects on IVF. PLoS ONE 8(3); e59062. DOI:10.1371/journal.pone.0059062, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0059062
- Sun, Z., Huang, S., Zhu, P., et al. (2022). Species-resolved sequencing of low-biomass or degraded microbiomes using 2bRAD-M. Genome Biology 23(1). DOI:10.1186/s13059-021-02576-9, https://genomebiology.biomedcentral.com/articles/10.1186/s13059-021-02576-9
- Hanna, A., Abbas, H., Yassine, F., et al. (2025). Systematic review of gut microbiota composition, metabolic alterations, and the effects of treatments on PCOS and gut microbiota across human and animal studies. Frontiers in Microbiology 16. DOI:10.3389/fmicb.2025.1549499, https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2025.1549499/full
- Senthilkumar, H., & Arumugam, M. (2025). Gut microbiota: a hidden player in polycystic ovary syndrome. Journal of Translational Medicine 23(1). DOI:10.1186/s12967-025-06315-7, https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06315-7
- Martinez Guevara, D., Vidal Cañas, S., Palacios, I., et al. (2024). Effectiveness of Probiotics, Prebiotics, and Synbiotics in Managing Insulin Resistance and Hormonal Imbalance in Women with Polycystic Ovary Syndrome (PCOS): A Systematic Review of Randomized Clinical Trials. Nutrients 16(22); 3916. DOI:10.3390/nu16223916, https://www.mdpi.com/2072-6643/16/22/3916
- Sola-Leyva, A., Perez-Prieto, I., Di Nisio, V., et al. (2025). Assessing the ovarian microbiome: lack of a distinguishable microbial signature beyond contamination. Reproductive BioMedicine Online 51(3); 104988. DOI:10.1016/j.rbmo.2025.104988, https://www.sciencedirect.com/science/article/pii/S1472648325001956
Further Reading
Last Updated: Aug 27, 2025