As IBD cases surge worldwide, scientists reveal how Western eating habits destabilise the gut–immune axis, and why tailored nutrition may be the key to stopping inflammation before it starts.
Review: Systems biology to unravel Western diet-associated triggers in inflammatory bowel disease. Image Credit: BlueRingMedia / Shutterstock
In a recent review in the journal Frontiers in Immunology, researchers discussed how global increases in inflammatory bowel disease (IBD) parallel the spread of Western lifestyle and dietary habits.
They concluded that Western dietary patterns, characterized by excessive salt, saturated and trans fats, ultra-processed foods, and refined sugars, significantly contribute to immune dysfunction, dysbiosis, and gut inflammation.
Combining nutritional research and systems biology could lead to the development of personalized preventive strategies and dietary therapies for IBD in rapidly Westernizing societies.
Westernisation and the Rise of IBD
The increasing prevalence of IBD is most marked in societies undergoing Westernisation, where chronic conditions like ulcerative colitis and Crohn’s disease present cycles of remission and flare-ups, involving both intestinal and systemic inflammation.
Diet and lifestyle changes, more than genetics, are driving the rapid global rise in IBD, with the Western diet, high in refined carbohydrates, omega-6-rich processed oils, ultra-processed foods, and salt, promoting inflammation and gut microbial disruption.
The rise in Western dietary habits coincides with increased incidence of other chronic inflammatory diseases, as evidence links these patterns to gut dysbiosis, loss of beneficial bacteria, and weakened intestinal barriers.
To better understand IBD’s multifactorial origins, researchers are increasingly turning to systems biology and nutritional epidemiology to integrate complex biological and environmental data, including early-life exposures such as maternal diet and breastfeeding practices.
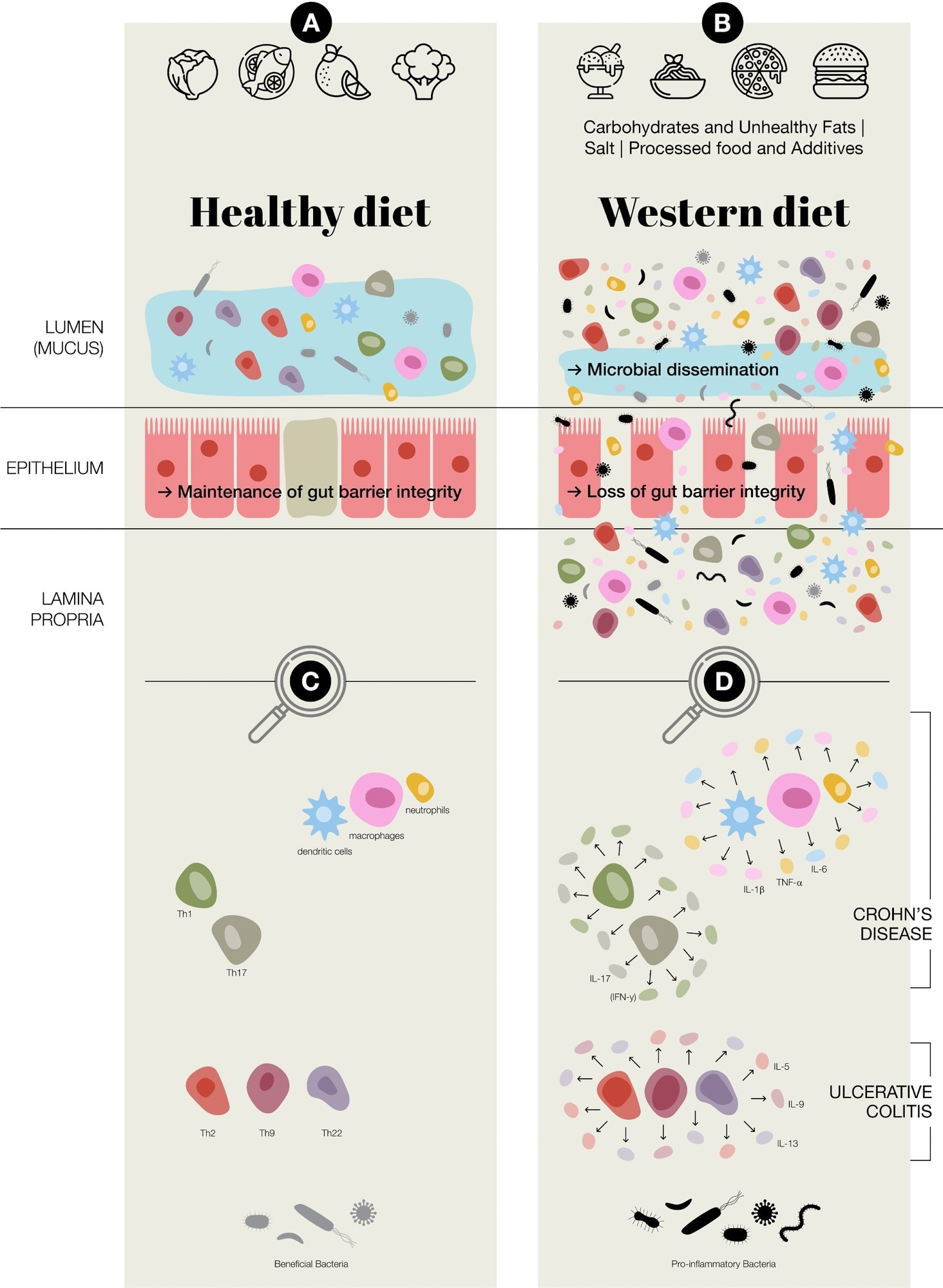
Potential of Western diet inducing chronic inflammation in the gut. (A) Healthy diet, maintenance of gut barrier integrity, normal immune regulation at the intestinal epithelial barrier, increased presence of beneficial bacteria; Faecalibacterium prausnitzii and Bifidobacterium (B) Western diet (Carbohydrates & Unhealthy Fats: alter gut microbiota, contribute to metabolic dysregulation and gut inflammation, increase intestinal permeability and endotoxemia. Processed food & Additives: disrupt gut microbial balance, erosion of the protective mucus layer, chronic low-grade inflammation. Salt: disrupting immune homeostasis, intestinal inflammation, alter the gut microbiota–immune system axis.); in general an aberrant activation of both innate and adaptive immune responses, loss of gut barrier integrity, a reduction in beneficial bacteria, such as Faecalibacterium prausnitzii and Bifidobacterium, and an increase in potentially pro-inflammatory species of bacteria, including adherent-invasive Escherichia coli and Ruminococcus gnavus (C) Predominate adaptive and innate immune cells present at intestinal epithelial barrier (D) Aberrant regulation/activation of predominate adaptive and innate immune cells in chronic inflammation in inflammatory bowel disease, which includes Crohn’s disease and ulcerative colitis.
Microbiota–Immune System Disruption
A healthy gut relies on a balanced interaction between the microbiota and the immune system. In IBD, this balance is disrupted, leading to chronic inflammation.
Dysbiosis, marked by reduced beneficial bacteria like Faecalibacterium prausnitzii and Bifidobacterium and increased harmful species such as E. coli, weakens gut barrier integrity and lowers short-chain fatty acid (SCFA) production. SCFAs, especially butyrate, typically strengthen epithelial cells and suppress inflammation.
When their levels fall, harmful metabolites such as hydrogen sulphide and trimethylamine-N-oxide (TMAO) increase, damaging tissues and sustaining inflammation.
The resulting increased intestinal permeability, sometimes referred to as “leaky gut” (a colloquial, non-diagnostic term), allows microbial components to penetrate the intestinal lining and activate immune pathways, increasing cytokine production.
Both innate and adaptive immunity become hyperactive, with Th1/Th17-skewed responses predominating in Crohn’s disease and Th2-biased signalling more evident in ulcerative colitis.
Understanding these interactions is essential for developing therapies aimed at restoring microbiota–immune equilibrium.
Dietary Drivers of IBD
Diet plays a central role in shaping gut health and influencing IBD risk. The Western diet’s excessive intake of refined carbohydrates, saturated and trans fats, and processed foods contributes to microbial imbalance, impaired barrier function, and immune activation.
High sugar intake increases intestinal permeability and oxidative stress, while unhealthy fats and processed meats promote pro-inflammatory bacterial growth and harmful metabolites.
Food additives, such as emulsifiers (e.g., carboxymethylcellulose), azo dyes, and artificial sweeteners, further disrupt the mucus layer and microbiota, aggravating inflammation. High salt intake reduces Lactobacillus spp., enhances IL-23 expression, and promotes Th17 activation, worsening immune imbalance and intestinal injury.
Conversely, fibre-rich diets foster beneficial bacteria and SCFA production, helping maintain barrier integrity and reducing inflammation. Identifying protective dietary components and reducing harmful ones could guide personalised nutrition strategies for preventing and managing IBD, supported by systems biology approaches that integrate dietary, microbial, and molecular data. However, researchers note that disentangling the effects of ultra-processing from overall nutrient content remains challenging, as ultra-processed foods are often high in fat and sugar, and low in fibre.
Systems Biology and Precision Nutrition
Recent advances in systems biology have transformed IBD research by integrating multi-omics data, including genomics, metagenomics, metabolomics, proteomics, and transcriptomics, to reveal microbial, genetic, and immune mechanisms underlying the disease.
While genome-wide association studies have identified over 200 IBD-related loci, genetics alone cannot explain disease variability, highlighting the influence of diet and environment.
Multi-omics studies have shown metabolic changes such as reduced SCFAs, bile acid imbalance, and oxidative stress. Emerging longitudinal analyses tracking remission and relapse phases offer insights for precision medicine, though integration challenges persist.
Statistical and network-based models, along with computational simulations, help visualise and predict disease mechanisms and therapy responses, but many of these remain hypothesis-generating and require rigorous clinical validation before translation into practice.
Meanwhile, nutritional epidemiology links high intake of ultra-processed foods with increased IBD risk, particularly at the highest consumption levels, whereas fibre- and polyphenol-rich Mediterranean diets show protective effects. Integrating systems biology with nutrition research promises personalised dietary interventions tailored to microbiome and immune profiles.
Conclusions and Future Directions
The rising global incidence of IBD mirrors Western dietary shifts marked by ultra-processed foods, excess sugar, and unhealthy fats. While genetics influences susceptibility, diet, microbiota imbalance, and immune dysregulation drive disease onset and progression.
Systems biology enables integrated analysis of multi-omic data to identify biomarkers, predict treatment response, and guide precision medicine. However, translating these findings into clinical practice requires standardised data pipelines and large longitudinal cohorts to establish causal relationships.
Nutritional epidemiology complements this approach by highlighting the preventive benefits of anti-inflammatory diets like the Mediterranean pattern. Future research should focus on precision nutrition informed by genomics and microbiome data, with artificial intelligence enhancing personalised dietary recommendations.
Understanding early-life nutritional exposures, such as maternal diet and infant feeding, as well as the effects of additives and emulsifiers on gut health, is also critical.
Interdisciplinary collaborations across gastroenterology, immunology, and nutrition are essential to shift IBD care from reactive treatment to individualised, proactive prevention strategies.
Journal reference:
- Konjar, S., Benedik, E., Šestan, M., Veldhoen, M., Županič, A. (2025). Systems biology to unravel Western diet-associated triggers in inflammatory bowel disease. Frontiers in Immunology, 16. DOI: 10.3389/fimmu.2025.1621334, https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2025.1621334/full