Introduction
What is BRCAness?
The Science Behind BRCAness
Clinical Significance of BRCAness
Challenges and Ongoing Research
Conclusion
References
Further Readings
Identifying BRCAness in tumors, even in the absence of BRCA mutations, broadens the reach of targeted therapies, supporting more individualized and effective cancer treatment approaches.
 Image Credit: Shutterstock.com
Image Credit: Shutterstock.com
Introduction
BRCAness describes traits in certain tumors, particularly sporadic ovarian cancers, that mimic cancers associated with inherited mutations in breast cancer gene 1 (BRCA1) or BRCA2. Although BRCA mutations primarily affect hereditary cancers, some tumors without these mutations also exhibit similar molecular disruptions, a phenomenon referred to as ‘BRCAness.’
Understanding BRCAness is critical because it highlights shared deficiencies in deoxyribonucleic acid (DNA) repair mechanisms, particularly homologous recombination. The presence of these mutations suggests that some cancers may be sensitive to treatments that damage DNA, including platinum-based chemotherapy and poly(ADP-ribose) polymerase inhibitors (PARPi). Thus, identifying BRCAness allows clinicians to identify patients who are most likely to benefit from targeted therapies, thereby improving treatment outcomes and advancing personalized cancer care.1
What is BRCAness?
BRCAness describes impaired homologous recombination repair (HRR) in certain cancer cells due to mutations or dysfunction in genes other than BRCA1 or BRCA2. Under normal conditions, BRCA1 and BRCA2 are essential for maintaining genomic stability by accurately repairing double-strand DNA breaks. When this repair mechanism fails, either due to BRCA mutations or similar disruptions in related genes, the risk of developing cancers, particularly those affecting breast and ovarian tissues, significantly increases.1,2
Understanding BRCAness is clinically important, as it reflects tumors with BRCA-like vulnerabilities, even in the absence of BRCA mutations, that could be targeted by therapies that exploit DNA repair weaknesses, particularly PARPi. By inducing synthetic lethality, PARPi selectively kills cancer cells with defective repair mechanisms.
BRCAness is not limited to breast and ovarian cancers, as tumors in the prostate, pancreas, and colon may also exhibit these traits. Thus, comprehensive screening for BRCAness can broaden the use of targeted therapies and support more effective, individualized cancer treatment strategies.1,2
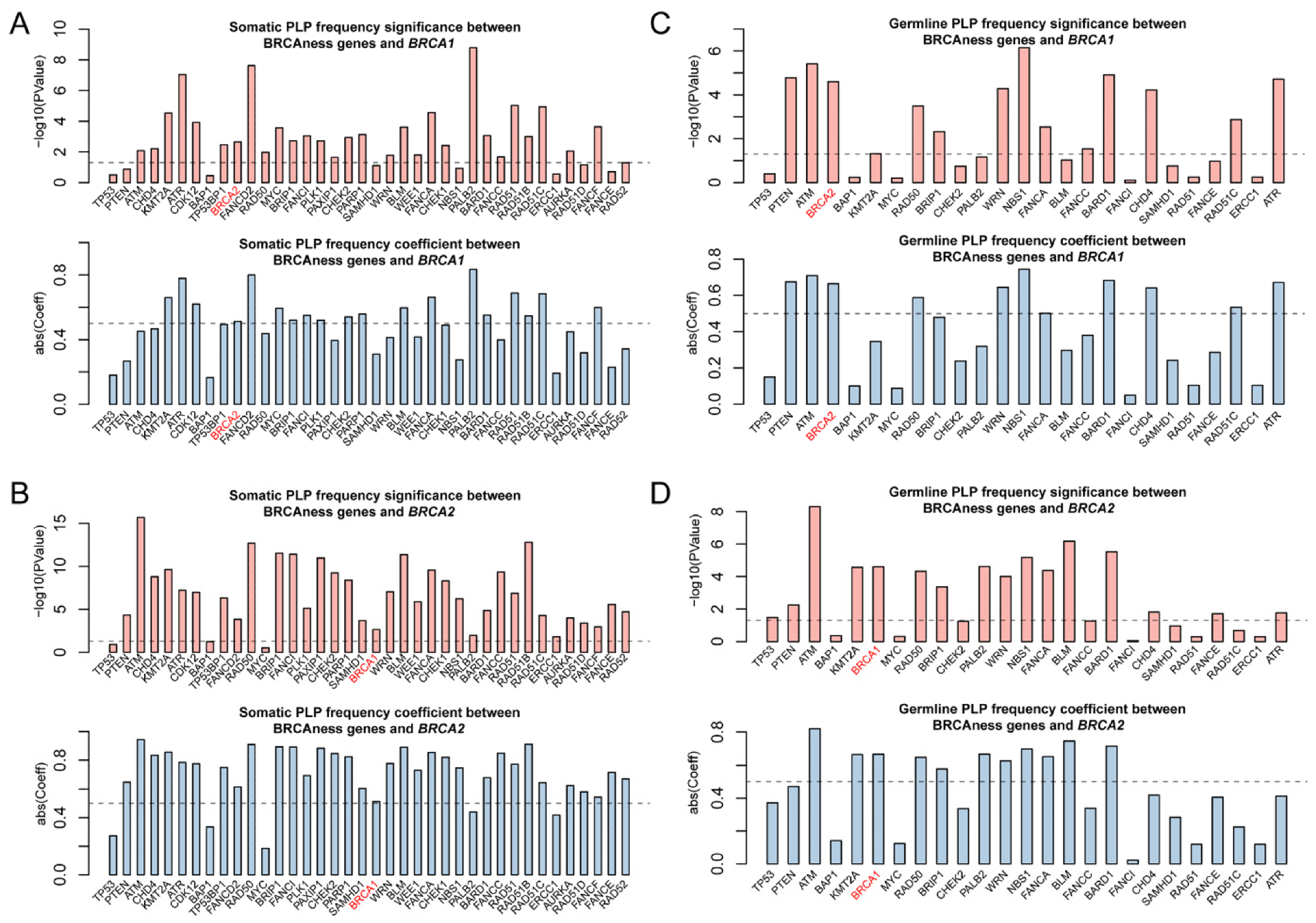 Correlation of pathogenic variation of BRCAness genes across 33 cancer types. (A,B) Significant correlation for the frequencies of somatic and germline pathogenic variants between the individual BRCAness gene and BRCA1. (C,D). Significant correlation for the frequencies of somatic and germline pathogenic variants between the individual BRCAness gene and BRCA2. Pairs with a p-value < 0.05 are regarded as significant. Pairs with an absolute coefficient greater than 0.5 are considered as significantly correlated.2
Correlation of pathogenic variation of BRCAness genes across 33 cancer types. (A,B) Significant correlation for the frequencies of somatic and germline pathogenic variants between the individual BRCAness gene and BRCA1. (C,D). Significant correlation for the frequencies of somatic and germline pathogenic variants between the individual BRCAness gene and BRCA2. Pairs with a p-value < 0.05 are regarded as significant. Pairs with an absolute coefficient greater than 0.5 are considered as significantly correlated.2
The Science Behind BRCAness
BRCA1 and BRCA2 genes are vital for maintaining genome stability through their role in HRR, a precise DNA double-strand break repair mechanism. In HRR, BRCA proteins help form radiation-sensitive 51 (RAD51)-single-stranded DNA (ssDNA) filaments, which are essential for invading and repairing damaged DNA using homologous sequences as templates.
This precise process is particularly important during the cell cycle phases when sister chromatids are available, primarily S and G2. Defects in BRCA genes impair HRR, thereby leading to genomic instability, a hallmark of cancer development.2,3
When BRCA1/2 functions are compromised, cells rely on error-prone alternative pathways, such as non-homologous end joining (NHEJ), which directly ligates broken DNA ends without using a homologous template. These defective repairs induce mutations, which subsequently promote cancer initiation and progression.2,3
BRCA Genes and Breast Cancer
BRCAness describes tumors that, despite lacking BRCA mutations, exhibit similar homologous recombination (HRR) deficiencies caused by mutations in other HRR-related genes, such as RAD51C, a partner and localizer of BRCA2 (PALB2), and BRCA1-interacting protein C-terminal helicase 1 (BRIP1), or through epigenetic modifications like promoter hypermethylation.
Tumors exhibiting BRCAness share distinct molecular features, including high-frequency C-to-T transitions, characteristic mutation signatures, and elevated promoter methylation levels. As a result, these tumors often respond to therapies targeting DNA repair weaknesses.2,3
Clinically recognizing BRCAness expands treatment opportunities beyond BRCA-mutated cancers. The tests that are used to detect BRCAness rely on genomic profiling, which provides crucial information on pathogenic mutations, homozygous deletions, epigenetic alterations, and gene expression patterns in relevant HRR and DNA repair genes.
The extensive genomic characterization of BRCAness across various cancer types reveals its widespread presence and potential therapeutic implications beyond breast and ovarian cancers. Prostate, colon, pancreatic, and endometrial cancers, for example, exhibit notable BRCAness features, which provides rationale for extending PARPi-based therapies to these malignancies.2,3
Clinical Significance of BRCAness
Tumors with BRCAness are clinically important because they respond well to treatments that exploit DNA repair defects, particularly PARPi and platinum-based chemotherapies. PARPi induces synthetic lethality by blocking DNA repair in cells that are already deficient, leading to the accumulation of unrepaired damage and ultimately, cancer cell death. Platinum drugs like cisplatin and carboplatin, which induce DNA cross-links, are associated with similar effectiveness against these tumors due to their compromised repair capacity.1,4,5
Recognizing BRCAness expands therapeutic options, thereby allowing more patients, beyond those with known BRCA mutations, to benefit from targeted treatments. For example, glioblastoma multiforme patients with biomarkers of BRCAness, such as mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2) or loss of phosphatase and tensin homolog (PTEN) function, have reported better outcomes when treated with PARPi.
Clinical studies have also highlighted how ovarian cancer patients with BRCAness traits experience improved responsiveness to platinum therapy, longer durations without treatment, and better survival rates as compared to patients lacking these characteristics.4,5
Likewise, clinical trials involving PARPi such as olaparib or niraparib have confirmed superior treatment outcomes in patients exhibiting BRCAness features, irrespective of actual BRCA mutations. Thus, accurately detecting BRCAness biomarkers can significantly refine treatment decisions, enhancing therapy effectiveness and advancing personalized cancer care.4,5
Challenges and Ongoing Research
Defining and applying the concept of BRCAness in the clinic presents several challenges, particularly for treating patients with glioblastoma and other heterogeneous tumors. One major obstacle is diagnostic uncertainty, as accurately identifying and measuring BRCAness is difficult due to the complexity of HRR pathways and overlapping biological roles of genes like BRCA1 and BRCA2.
BRCAness may result from various genetic and epigenetic alterations, including mutations in HRR-related genes such as IDH1/2, PTEN, as well as DNA damage response genes like ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR).5,6
Currently available diagnostic tools, such as gene sequencing panels, HR deficiency (HRD) scores based on genomic instability indicators, as well as functional assays like RAD51 foci formation, are either limited in accuracy, not broadly standardized, or remain experimental. For example, FoundationOne CDx sequences 324 cancer-associated genes; however, it remains challenging to distinguish true deleterious mutations from those that are non-pathogenic .5,6
Tumor heterogeneity further complicates BRCAness detection. Variability in gene expression, DNA repair activity, and the tumor microenvironment across patients and cancer types undermines efforts to apply a universal definition. Alternative DNA repair mechanisms may also mask homologous recombination defects, thereby reducing test reliability.5,6
Ongoing research aims to refine biomarkers of BRCAness and validate tissue-specific HRD signatures using transcriptional profiling, promoter methylation patterns, and RNA sequencing. The ultimate goal is to improve patient stratification and guide precision therapy with PARPi, such as olaparib and niraparib.5,6
Conclusions
BRCAness plays a vital role in advancing personalized cancer treatment by identifying tumors with DNA repair defects similar to those observed in BRCA-mutated cancers. Despite the diagnostic challenges posed by tumor complexity, ongoing research aims to improve detection methods and validate new biomarkers.
As our understanding deepens, BRCAness offers hope for more precise and effective cancer therapies tailored to individual tumor biology, ultimately enhancing outcomes and reshaping the future of cancer care.
References
- Rigakos, G., & Razis, E. (2012). BRCAness: finding the Achilles heel in ovarian cancer. The oncologist, 17(7), 956-962. DOI: 10.1634/theoncologist.2012-0028, https://academic.oup.com/oncolo/article/17/7/956/6403273
- Guo, M., & Wang, S. M. (2022). The BRCAness landscape of cancer. Cells, 11(23), 3877. DOI: 10.3390/cells11233877, https://www.mdpi.com/2073-4409/11/23/3877
- Gorodetska, I., Kozeretska, I., & Dubrovska, A. (2019). BRCA genes: the role in genome stability, cancer stemness and therapy resistance. Journal of Cancer, 10(9), 2109. DOI: 10.7150/jca.30410, https://www.jcancer.org/v10p2109.htm
- Xavier, M. A., Rezende, F., Titze-de-Almeida, R., & Cornelissen, B. (2021). BRCAness as a biomarker of susceptibility to PARP inhibitors in glioblastoma multiforme. Biomolecules, 11(8), 1188. DOI: 10.3390/biom11081188, https://www.mdpi.com/2218-273X/11/8/1188
- Muggia, F., & Safra, T. (2014). ‘BRCAness’ and its implications for platinum action in gynecologic cancer. Anticancer research, 34(2), 551-556. https://pmc.ncbi.nlm.nih.gov/articles/PMC4682661/
- Murai, J., & Pommier, Y. (2023). BRCAness, homologous recombination deficiencies, and synthetic lethality. Cancer research, 83(8), 1173-1174. DOI: 10.1158/0008-5472.CAN-23-0628, https://aacrjournals.org/cancerres/article/83/8/1173/725094/BRCAness-Homologous-Recombination-Deficiencies-and
Further Reading
Last Updated: Jun 1, 2025