Understanding epigenetics
Epigenetics and development
Epigenetics and disease
Epigenetics and lifestyle
References
Further reading
Epigenetics plays a critical role in human health by regulating gene expression without the direct modification of DNA sequence. Aberrant epigenetic modifications mediated by changes in DNA and histone methylation and acetylation patterns, in addition to non-coding RNAs, can disrupt normal cellular function and lead to chronic conditions such as cancer and neurological disorders.

Image Credit: VectorMine/Shutterstock.com
Understanding epigenetics
Epigenetic modifications induce or suppress gene expression without altering the DNA sequence.1 Such modifications include DNA methylation, post-translational histone modifications, and gene expression regulation by ncRNAs.1
The histone proteins, in conjunction with the chromatin, constitute the nucleosomes.1 When the N-terminal tails of histone undergo acetylation or methylation, alterations in chromatin structure and function are observed, which subsequently impact gene expression.1
A variety of extrinsic and intrinsic environmental factors, including nutrition, exposure to toxins, inflammation, aging, exercise, medication, social stress, and metabolic or hormonal disorders, have been linked to influence epigenetic patterns in germ cells.2
These modifications can then be inherited by subsequent generations.2 When epigenetic modifications are transmitted from parents to their direct offspring, it is referred to as intergenerational epigenetic inheritance.2 When these modifications persist beyond the first generation, it is defined as transgenerational epigenetic inheritance.2
The scientific community continues to debate the existence of transgenerational epigenetic inheritance in mammals.2 However, the postulated mechanisms of epigenetic inheritability involve DNA methylation, which commonly acts to repress gene expression, and histone modifications, which can either activate or repress gene expression but are often context-dependent.1-2
Non-coding RNAs, on the other hand, are involved in both transcriptional and post-transcriptional regulation of gene expression, as well as chromatin remodeling through ncRNA-dependent recruitment of chromatin remodeling complexes.1,3
It is noteworthy that research has demonstrated the capacity of acquired epigenetic signatures at specific regions of the mouse genome to be inherited across generations.1 Additionally, during gametogenesis, not all methylated cytosines (5-mC) in primordial germ cells undergo demethylation; a significant proportion of these epigenetic marks exhibit resistance to complete erasure.1
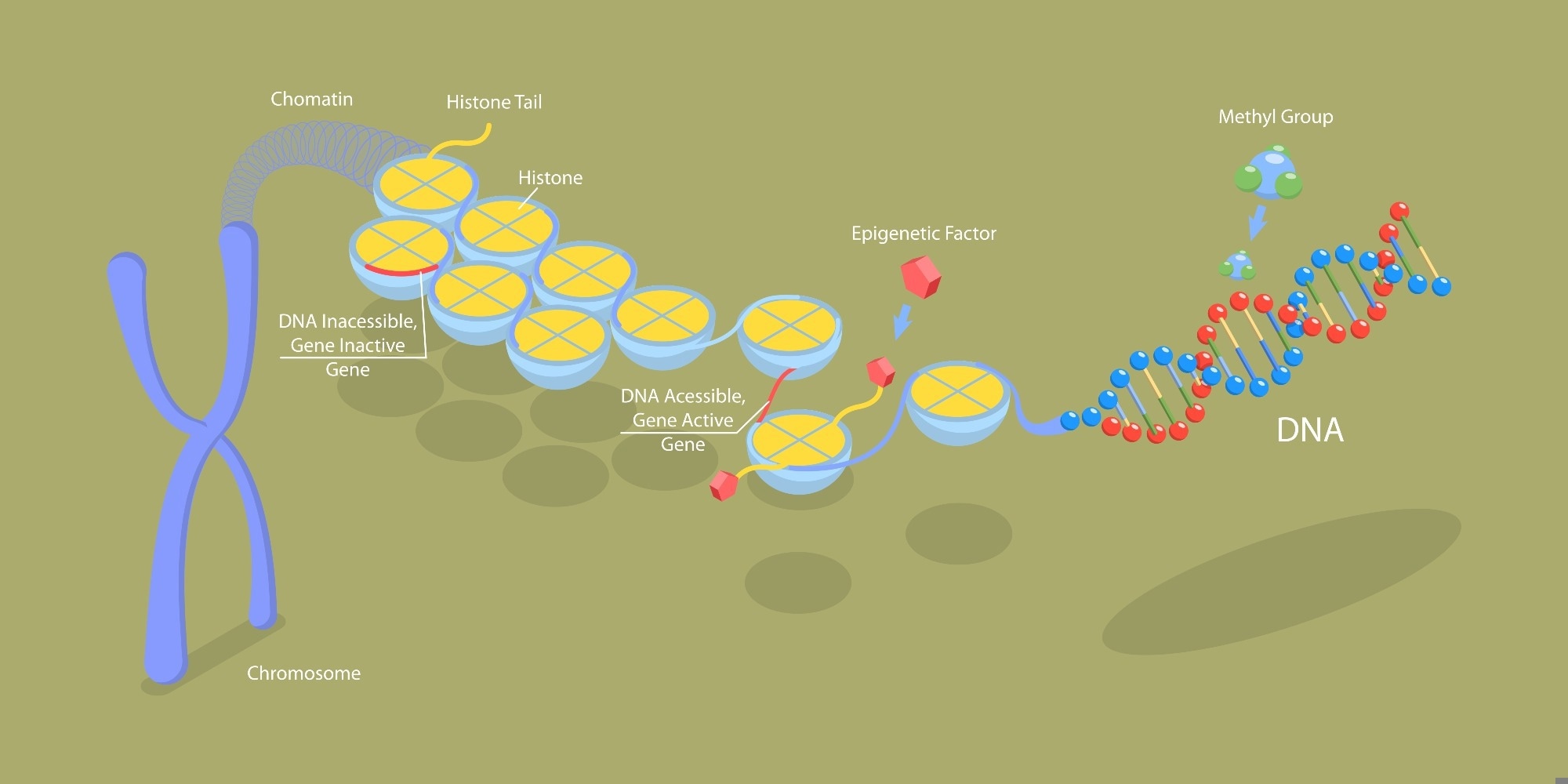
Image Credit: TarikVision/Shutterstock.com
Approximately 40% of 5-mC and its derivatives persist following extensive epigenetic reprogramming during meiosis.1 Consequently, sperm and oocytes retain an important part of the parental DNA methylation patterns.1
Epigenetics and development
During human embryogenesis, chromatin undergoes extensive remodeling, altering its accessibility at key developmental stages.4 These changes support gene regulatory network rewiring and the establishment of new developmental programs. Regions gaining accessibility are mainly compromised in promoters, CpG islands, and enhancers.4
Over 8,000 promoters open in zygotes, remaining accessible through preimplantation; these genomic regions are enriched with specific metabolic and biosynthetic functions.4
It is important to note that epigenetics plays a significant role in both embryo development and the development of primordial germ cells (PGC), which are precursors of the embryo.5
During their development, mammalian PGCs undergo a distinctive and comprehensive epigenetic remodeling process, coinciding with their transition toward totipotency.5 It is observed that there are differences in the rates of OXPHOS and glycolysis, as well as sexual dimorphism.5
In order to ensure genomic stability during the differentiation process, certain genomic regions retain higher DNA methylation, resisting global demethylation.5 Histone remodeling involves changes in the methylation patterns of H3K9me2, H3K27me3, and H2A/H4R3me2s. These changes are coordinated to safeguard the genome during the transition to totipotency.5
Gene expression is also affected by ncRNAs that interfere with transcription by modulating chromatin. They play roles in dosage compensation and imprinting, crucial for normal development through epigenetic chromatin state modulation.6
Environmental factors that act during the process of embryogenesis, including dietary intake and exposure to toxins, have the potential to alter the uterine environment and fluid composition.7 This, in turn, can affect epigenetic modifications in embryos, influencing their development and potentially leading to structural and functional alterations in the offspring.7
These changes can impact systems such as the immune and cardiovascular systems.7 The effect of these factors causes important metabolic and endocrine changes that can predispose the fetus to certain postnatal diseases.8 This concept is known as fetal programming.8
Epigenetics and disease
A variety of studies have highlighted the significant role of environmental factors in influencing epigenetic mechanisms, contributing to the pathophysiology of different diseases.
Exposure to neurotoxins, pesticides, and heavy metals has been shown to alter epigenetic patterns linked to Parkinson's disease (PD).9 These changes include increased histone acetylation and DNA methylation, affecting genes associated with PD such as PINK1, PARK2 and TH.9
Hypermethylated patterns have been identified in tumors, frequently located in gene promoter regions of tumor suppressor genes, in contrast to the overall hypomethylated regions observed in cancer cells.10 These hypermethylation patterns have been linked to the development of breast cancer, liver cancer, prostate cancer, and small-cell bladder cancer.10
Additionally, dysregulation of histone deacetylases has also been observed in various cancers, as well as the influence of ncRNAs effect on the expression of protooncogenes like c-Myc in colorectal cancer (CRC).10
Epigenetics plays a crucial role in major depressive disorder and schizophrenia research, with DNA methylation significantly influenced by environmental stressors like childhood adversity.11
These stressors lead to lasting methylation changes, affecting neuroendocrine responses, neuroplasticity, neurotransmission, and neural development in both brain and peripheral tissues.11
Epigenetics
Histone modifications, especially lysine acetylation, are linked to stress responses and antidepressant effects in depression.11 Additionally, ncRNAs, including circRNAs, miRNAs, and lncRNAs, regulate gene expression, impacting synaptic transmission, insulin resistance, immune responses, and inflammation in these disorders.11
Epigenetics and lifestyle
Diet, exercise, and stress significantly impact the epigenome by influencing DNA methylation, histone modifications, and ncRNAs.12
A healthy diet and regular exercise promote beneficial epigenetic changes, such as DNA demethylation and histone modifications, which can help prevent chronic diseases like cancer, diabetes, and cardiovascular disorders.12
Conversely, chronic stress can lead to detrimental epigenetic changes, contributing to disease progression.12 Early-life nutrition is crucial for inducing lifelong epigenetic modifications, while regular physical activity and stress management are essential for maintaining positive epigenetic health.12
Epigenetic therapies hold promise for treating diseases, including cancer and neurodegenerative disorders.13 The reversible nature of the epigenome allows for reprogramming through environmental and pharmacological interventions, enhancing therapeutic strategies.13
However, ethical implications, such as privacy concerns and potential discrimination based on epigenetic profiles, necessitate careful consideration in research and clinical applications, emphasizing the need for equitable access and public policy measures.14
References
- Trerotola, M., Relli, V., Simeone, P., & Alberti, S. (2015). Epigenetic inheritance and the missing heritability. Human Genomics, 9(1). https://doi.org/10.1186/s40246-015-0041-3
- Khatib, H., Townsend, J., Konkel, M. A., Conidi, G., & Hasselkus, J. A. (2024). Calling the question: what is mammalian transgenerational epigenetic inheritance? Epigenetics, 19(1). https://doi.org/10.1080/15592294.2024.2333586
- Kaikkonen, M. U., Lam, M. T. Y., & Glass, C. K. (2011). Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovascular Research, 90(3), 430–440. https://doi.org/10.1093/cvr/cvr097
- Wilkinson, A. L., Zorzan, I., & Rugg-Gunn, P. J. (2023). Epigenetic regulation of early human embryo development. Cell Stem Cell, 30(12), 1569–1584. https://doi.org/10.1016/j.stem.2023.09.010
- Verdikt, R., & Allard, P. (2021). Metabolo-epigenetics: the interplay of metabolism and epigenetics during early germ cells development†. Biology of Reproduction, 105(3), 616–624. https://doi.org/10.1093/biolre/ioab118
- Pauli, A., Rinn, J. L., & Schier, A. F. (2011). Non-coding RNAs as regulators of embryogenesis. Nature Reviews Genetics, 12(2), 136–149. https://doi.org/10.1038/nrg2904
- Lamberto, F., Peral-Sanchez, I., Muenthaisong, S., Zana, M., Willaime-Morawek, S., & Dinnyés, A. (2021). Environmental Alterations during Embryonic Development: Studying the Impact of Stressors on Pluripotent Stem Cell-Derived Cardiomyocytes. Genes, 12(10), 1564. https://doi.org/10.3390/genes12101564
- Kwon, E. J., & Kim, Y. J. (2017). What is fetal programming?: a lifetime health is under the control of in utero health. Obstetrics & Gynecology Science, 60(6), 506. https://doi.org/10.5468/ogs.2017.60.6.506
- Tsalenchuk, M., Gentleman, S. M., & Marzi, S. J. (2023). Linking environmental risk factors with epigenetic mechanisms in Parkinson's disease. Npj Parkinson S Disease, 9(1). https://doi.org/10.1038/s41531-023-00568-z
- Yu, X., Zhao, H., Wang, R., Chen, Y., Ouyang, X., Li, W., Sun, Y., & Peng, A. (2024). Cancer epigenetics: from laboratory studies and clinical trials to precision medicine. Cell Death Discovery, 10(1). https://doi.org/10.1038/s41420-024-01803-z
- Yuan, M., Yang, B., Rothschild, G., Mann, J. J., Sanford, L. D., Tang, X., Huang, C., Wang, C., & Zhang, W. (2023). Epigenetic regulation in major depression and other stress-related disorders: molecular mechanisms, clinical relevance and therapeutic potential. Signal Transduction and Targeted Therapy, 8(1). https://doi.org/10.1038/s41392-023-01519-z
- Epigenetics and Diet: How Food Influences Gene Expression. (2024, June 26). Rupa Health. https://www.rupahealth.com/post/epigenetics-and-diet-how-food-influences-gene-expression
- Majchrzak-Celińska, A., Warych, A., & Szoszkiewicz, M. (2021). Novel Approaches to Epigenetic Therapies: From Drug Combinations to Epigenetic Editing. Genes, 12(2), 208. https://doi.org/10.3390/genes12020208
- Santaló, J., & Berdasco, M. (2022). Ethical implications of epigenetics in the era of personalized medicine. Clinical Epigenetics, 14(1). https://doi.org/10.1186/s13148-022-01263-1
Further Reading
Last Updated: Sep 23, 2024