Breast cancer is a major concern throughout the world. It is the number 1 cancer among women and in the Western world it generates approximately one new case per minute: 24 hours a day 7 days a week.
It is a disease that is often managed fairly well if caught early but it has an insidious nature about it – it can recur after as many as 10-15 years. Once it is metastatic, our ability to treat it is very diminished.
How many people does breast cancer affect?
There are about 600,000 new cases each year in the world but from a societal point of view, because women are so critical in our families, in our relationships and really are the ‘fabric’ of society it affects a much larger network. When a woman gets breast cancer, her entire extended family and community are affected.
What tests are currently used to detect breast cancer?
We do reasonably well at detecting breast cancer when it is fairly large and half way through its cycle. The methods include mammography, ultrasound, magnetic resonance imaging (MRI), and clinical breast exams.
All of these tests as I’ve indicated are breast cancer tests. There are some new tests on the horizon like the ForeCYTE Breast Health test that is actually designed not to pick up cancer but to pick up the steps before cancer – the precancerous stage. At this stage it is much easier to treat and reverse the changes because by-definition it is reversible up to the point when it becomes cancer.
What are the limitations of these tests?
With the mammogram one of the key limitations that we don’t talk about very much is that it uses X-ray radiation and we know that X-ray radiation causes cancer. Some people estimate as many as 36,000 cancers in the community of women are caused by the radiation from mammography. So that’s its largest drawback other than the fact that they aren’t very good at small lesions and they don’t do anything for pre-cancers – i.e. they don’t find the things that lead to cancer.
It takes about 10 years for pre-cancer changes to become cancers so we are missing that entire window of opportunity.
Please can you tell us about the new test developed by Atossa Genetics?
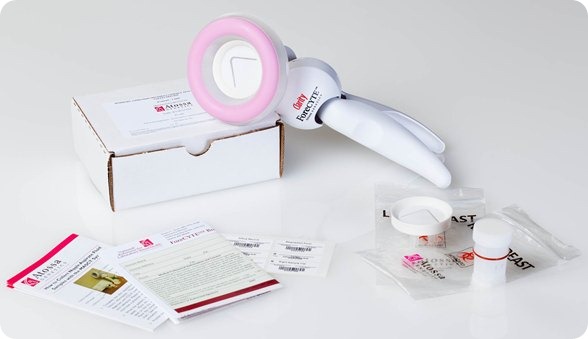
The ForeCYTE Breast Health test, which we are launching nationally with our partner Clarity Women’s Health, is literally a Pap smear for breast cancer.
As you may know the Pap smear is a test for cervical cancer and it takes a small scraping from the cervix and looks at the changes under the microscope and can see pre-cancer changes up to 10 years before cervical cancer appears. That test is the most successful screening test in all of medicine – it has led to an 80% reduction in cervical cancer in the population. It’s now almost malpractice for a woman to get cervical cancer if she is having regular Pap smears.
We’ve never had the opportunity to have that kind of test with the breast until now and we’re really excited to be able to offer a similar test for breast health.
What stage of development is this test currently at?
It has gone through all of the FDA clearance process, which is a multi-year, multi-million dollar process. We are manufacturing it here in the States and it was launched last year in a soft, small launch among doctors in America.
We now are in the process of doing a national launch in the US and we will bring it out overseas towards the end of this year or the beginning of next year.
What are the benefits of this test?
Because it is a test of pre-cancer not cancer, it gives us an entirely new opportunity to think about how to manage breast health in the medical community. Because breast cancer, like cervical cancer, takes 10 years to develop; being able to look at that process at year 1 or year 2 gives us many options for intervening.
As I’ve indicated before, the pre-cancerous state is highly susceptible to reversion back to normal.
Can you explain how the test works?
The test involves a nurse, or a doctor, or a physician’s assistant using a modified breast pump (the kind of pump that women use when they are breastfeeding their child) to collect a tiny sample from the nipple of each non-lactating woman and then the sample is processed at our lab for genetic changes and we look at it under the microscope. We then write a report back to the doctor.

It takes less than 10 minutes in total. It takes around 1 minute with each breast to collect the specimen. It is painless – it caused much lower levels of pain in the clinical trials than the mammogram or even than breast feeding a child.
It’s quick, convenient and there is no radiation. It is also easy to use in the doctor’s office.
Can the test be used on men?
That is an interesting question and we haven’t actually looked at it. As you’ve indicated men do get breast cancer, it is about 1% of the rate of women and so it is just something we haven’t gotten to.
It seems like it should work on men, but we have not done any work on it at all.
What impact do you think this test will have?
Since the pap smear lowered cervical cancer by 80% because doctors intervene in the pre-cancerous process – that’s our goal. We want to lower breast cancer rates by 80%.
How do you think the future of breast cancer testing will develop?
If we can get this test widely adopted, it will change the paradigm. In the new paradigm, we would not be looking for cancer and then doing the current ‘cut, burn and poison’ routine we would be doing something much gentler with the pre-cancerous condition.
Do you think it will be possible to prevent breast cancer completely one day?
Yes, that’s our goal.
What are Atossa Genetics’ plans for the future?
We have 3 other tests in the CarePath of breast health. If a woman is identified as at high risk with the ForeCYTE test we have another test to determine which segment of the breast is undergoing pre-cancerous changes. The breast genetically is divided into 7 different segments on average. So our second test called the FullCYTE test determines which of the 7 segments is undergoing pre-cancerous changes, because we’d like to treat just those segments, again before a woman gets breast cancer, with an intraductal therapy.
We also have a test for women who have breast cancer where we look at the tumor and determine what drugs should be best used to treat it.
We have a survivors test which is a blood test looking for the late process – circulating tumor cells – again to intervene before they become metastatic.
Four tests and a pre-cancerous treatment plan keep us busy!
Why is 2013 a big year for Atossa Genetics?
I just want to emphasise that 2013 is a very big year for us with the national launch of the ForeCYTE test and the movement of the other tests into the market. So for all of our stakeholders – patients, doctors, and investors – this will be a seminal year for Atossa Genetics.
Where can readers find more information?
https://atossatherapeutics.com/
About Dr Steven Quay
 Dr. Quay has served as Chief Executive Officer and Chairman of the Board of Directors of Atossa Genetics since the Company was incorporated in April 2009.
Dr. Quay has served as Chief Executive Officer and Chairman of the Board of Directors of Atossa Genetics since the Company was incorporated in April 2009.
Prior to his work at Atossa Genetics, Dr. Quay served as Chairman of the Board, President and Chief Executive Officer of MDRNA, Inc. from August 2000 to May 2008, and as its Chief Scientific Officer until November 30, 2008 (MDRNA, Inc. was formerly known as Nastech Pharmaceutical Company Inc. and is currently known as Marina Biotech, Inc.). From December 2008 to April 2009, Dr. Quay was involved in acquiring the Company’s assets and preparing the Company’s business plan.
Dr. Quay is certified in Anatomic Pathology with the American Board of Pathology, completed both an internship and residency in anatomic pathology at the Massachusetts General Hospital, a Harvard Medical School teaching hospital, is a former faculty member of the Department of Pathology, Stanford University School of Medicine, and is a named inventor on 14 U.S. and foreign patents covering the MASCT System.
He oversaw the clinical testing and regulatory filing of the MASCT device with the FDA that led to its ultimate marketing clearance. Including the patents for the MASCT System, Dr. Quay has a total of 76 U.S. patents, 98 pending patent applications and is a named inventor on patents covering five pharmaceutical products that have been approved by the FDA.
Dr. Quay received an M.D. in 1977 and a Ph.D. in 1975 from the University of Michigan Medical School. He also received his B.A. degree in biology, chemistry and mathematics from Western Michigan University in 1971.
Dr. Quay is a member of the American Society of Investigative Pathology, the Association of Molecular Pathology, the Society for Laboratory Automation and Screening and the Association of Pathology Informatics.