Feb 13 2015
Imagine not being able to climb the stairs without stopping to take a break, or getting winded trying to stand and do the dishes. Unfortunately these are common realities for individuals suffering from respiratory conditions. Ventilators can help reduce the work of breathing by unloading the ancillary respiratory muscles, but they are often bulky and heavy, creating additional limitations for users. The Breathe Non-Invasive Open Ventilation (NIOV) System technology is a better solution. By reducing the overall size of the ventilation system, it supports patient mobility and independence.
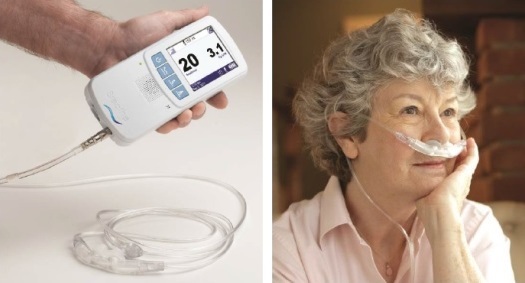
Makrolon® polycarbonate from Bayer MaterialScience LLC lends its outstanding properties and light weight to help Breathe Technologies Inc. achieve the high-performing yet portable device design.
Makrolon® polycarbonate from Bayer MaterialScience LLC lends its outstanding properties and light weight to help Breathe Technologies Inc. achieve the highperforming yet portable device design. The Breathe NIOV System is a onepound, non-invasive mechanical ventilator that can be used in home and institutional settings. Breathe Technologies explains that the device utilizes novel venturi principle technology in a comfortable facial interface that can be worn while talking and exercising. The patient interface and air/oxygen path components utilize injection-molded Makrolon® 2858 polycarbonate. This grade is suitable for ETO and steam sterilization at 121 C and is biocompatible according to many ISO 10993-1 test requirements, making it an ideal choice for medical devices. Makrolon® 2858 polycarbonate also features easy release and medium viscosity and is available in transparent and opaque colors. The device components made of Makrolon® polycarbonate are intricate in design and require optimal functionality for patient safety. Choosing the right material was key to the success of the device.
“We wanted to create a durable and comfortable patient interface, and Makrolon® polycarbonate was the ideal material for our design and intended use,” explains Larry Mastrovich, president and CEO, Breathe Technologies. “The material’s high quality, biocompatibility and proven performance, along with the support of Bayer’s experts, solidified our choice.” “We worked collaboratively with Breathe Technologies to ensure they found the right material for their application,” says Richard Aldrich, field market development manager, Bayer MaterialScience LLC. “By providing samples, technical data and medical submission support, as well as answering biocompatibility questions, we were able to help Breathe make the right material choice.”
According to Breathe Technologies, the Breathe NIOV System is the first and only wearable ventilation system that has been cleared by the U.S. FDA for use in patients with respiratory insufficiency, chronic obstructive pulmonary disease (COPD), including Alpha-1 Antitrypsin Deficiency, and in patients with neuromuscular diseases. The Breathe NIOV System has received five 510(K) clearances from the U.S. FDA for use with compressed oxygen or compressed air for home and institutional use, and includes invasive and noninvasive circuits. Additionally, Breathe Technologies has found through clinical studies and clinician feedback that the device has been clinically impactful.
As the nation’s health care needs increase, so do medical technology advancements. Materials must keep up to meet the demanding needs of medical applications. “Bayer works to create materials that are of the highest quality, and that can be used in a multitude of life-changing products,” said Bruce Fine, market segment leader – medical, North America, Bayer MaterialScience LLC. “Our involvement with Breathe Technologies to bring this device to market underscores Bayer’s commitment to staying at the forefront of technology and changing peoples’ lives for the better.”