New research reveals that while losing weight can rejuvenate aging fat tissue, stubborn immune cells keep a “memory” of obesity, offering fresh targets for lasting metabolic health.
 Study: Selective remodelling of the adipose niche in obesity and weight loss. Image Credit: Andrey_Popov / Shutterstock
Study: Selective remodelling of the adipose niche in obesity and weight loss. Image Credit: Andrey_Popov / Shutterstock
In a recent study published in the journal Nature, researchers utilized next-generation assays to investigate the regulatory factors, cell types, and molecular events involved in human adipose tissue (AT) remodeling. Their landmark study provides the most detailed view yet into how fat tissue transforms during obesity and weight loss.
Study findings reveal that obesity induces cellular senescence, inflammation, and structural stress within fat, which are only partially reversed by weight loss. Notably, senescence reversal was selective, occurring in metabolic adipocytes, vascular cells, and precursors where p21-positive senescent cells were nearly eliminated, but largely persistent in immune cells. In combination with the 171,247-cell strong atlas, these findings provide a revolutionary understanding of fat tissue plasticity, representing the first step towards novel therapeutic directions that may finally overcome obesity's notorious capacity to bounce back.
Background
Obesity is a metabolic condition characterized by excessive systemic fat accumulation, with potentially lethal outcomes. Clinically defined as a body mass index (BMI) > 30 kg/m², obesity has been linked to several chronic diseases, including type 2 diabetes (T2D), cardiovascular diseases (CVDs), and even certain cancers.
Alarmingly, more than 1 billion humans live with obesity, with today's suboptimal lifestyle expected to exacerbate this already grim situation. While pharmacological, behavioral, and surgical interventions have demonstrated promise in achieving weight loss, these benefits are often temporary.
These facts reveal our surface understanding of the association and correlation between weight loss and improved obesity-associated health outcomes, while emphasizing our lack of knowledge of the mechanistic interplay governing fat (adipose tissue [AT]) remodeling.
About the Study
The present study aims to address this knowledge gap and progress our war on obesity by deciphering the cellular, molecular, and spatial changes that occur in human AT during obesity and after weight loss.
Study samples (subcutaneous abdominal AT) were obtained from morbidly obese patients (BMI > 35 kg/m²; n = 25; age = 20–70 yrs) and healthy controls (BMI < 26 kg/m²) intra-operatively via laparoscopic bariatric surgery. Follow-up samples were collected five months later for comparative assays. All samples were subjected to single-nucleus RNA sequencing (snRNA-seq) using the Illumina NextSeq2000 platform.
In brief, the study profiled ~100,000 nuclei from fresh biopsies and integrated the resultant sequences with 50,000 additional nuclei from the largest human fat tissue atlas to date. To supplement this single-cell data atlas, the 10x Genomics' Xenium platform was leveraged to conduct spatial transcriptomics on over 25,000 cells from equivalent cohorts, allowing researchers to observe differences in tissue-specific cell states.
Simultaneously, formalin-fixed paraffin-embedded (FFPE) AT samples were subjected to 5 μm sectioning, 4',6-diamidino-2-phenylindole (DAPI) staining, and in situ hybridization assays, allowing researchers to both carry out nuclear identification and measure gene activity directly within intact tissue architecture.
Finally, the study used pathway enrichment (MSigDB), differential expression, transcription factor inference, and spatial niche modeling assays to elucidate the movement, activity patterns, and remodellings of metabolic, immune, vascular, and progenitor cell populations.
Study Findings
The present study makes five key findings:
-
Weight loss selectively reverses senescence in metabolic, vascular, and precursor cells
Metabolic adipocytes, precursor cells, and vascular cells of patients with obesity were found to express senescence-associated secretory phenotype (SASP) genes and other markers of cellular senescence. These cells contribute to fibrosis, immune signaling, and local tissue stress.
Notably, following weight loss (bariatric surgery), the fraction of "stressed" adipocytes (AD3) was observed to decrease dramatically (from 55% to 14%; P < 0.0001), and p21-positive senescent cells were nearly eliminated in these lineages. However, senescence persisted in immune cells (macrophages), suggesting only partial and cell-type-specific rejuvenation of the adipose niche.
-
Immune cells retain inflammatory programming post-weight loss
While the infiltration of lipid-associated macrophages (LAMs) was observed to decrease following weight loss, these LAMs retained inflammatory signature (TREM1 and TLR2) expression, indicating transcriptional memory that may predispose to metabolic dysfunction and potentially explain obesity's frequent reappearance.
-
Adipocyte metabolism globally activates during weight loss but fails to restore lean-state flexibility
Weight loss enhanced substrate cycling (e.g., triglyceride hydrolysis/resynthesis) and branched-chain amino acid breakdown, but metabolic parameters (e.g., insulin sensitivity) remained suboptimal relative to lean baselines.
-
Weight loss reduces structural tissue stress and fibrosis
Weight loss was observed to substantially downregulate the expression of genes (e.g., LOX, ACTA2, and VGLL3) involved in fibrosis and cytoskeletal tension, resulting in reduced adipocyte stiffness and inflammation.
-
Spatial mapping reveals stress niches adjacent to immune hotspots
The study identified five functional tissue niches: adipocyte, vascular, stress, arterial, and stem-like zones. Stressed cells formed distinct "stress niches" adjacent to immune-enriched arterial zones, indicating microenvironments of obesity-caused damage with delayed recovery. Several stress-associated factors (THBS1, NAMPT, AREG) were found to be enriched in obesity and reversed by weight loss (P < 0.05), highlighting them as potential therapeutic targets.
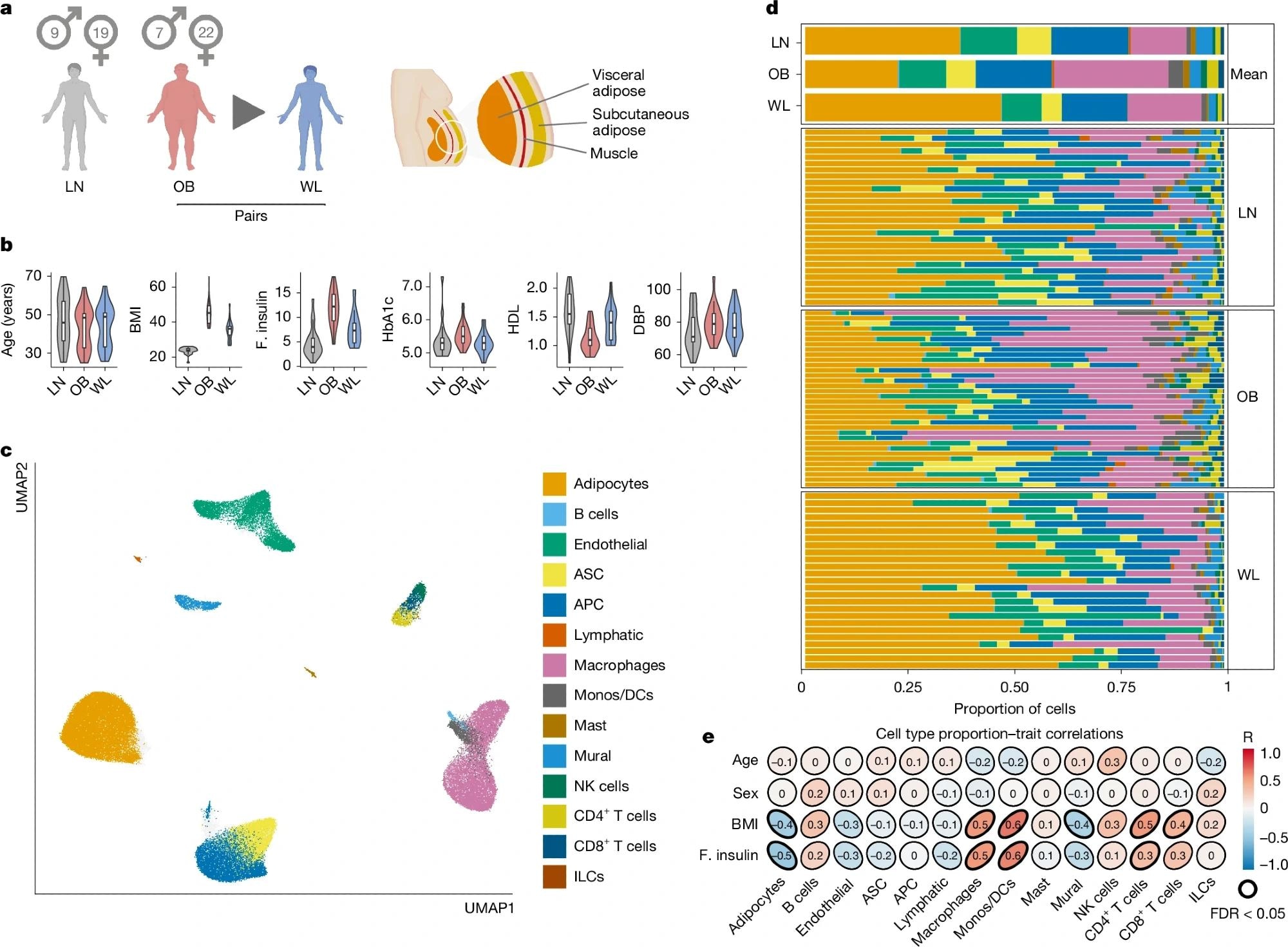 a, Graphical representation of the primary study cohort (left; single-nucleus analyses in n = 25 obese (OB) people before and after WL and n = 24 lean (LN) people, with spatial analyses in n = 4 people per group) and AT anatomical location (right). b, Clinical characteristics of the primary cohort (n = 24 LN and 25 paired OB–WL donors). Boxplot, median interquartile range minimum and maximum. BMI, body mass index (kg m–2); F insulin, fasting insulin (mIU L–1); HbA1c, haemoglobin A1c (%); HDL, high-density lipoprotein cholesterol (mM); DBP, diastolic blood pressure (mm Hg). c, Uniform manifold approximation and projection (UMAP) of 145,452 human AT cells (n = 74 samples of the primary cohort and n = 13 samples of the Emont published cohort11, single nucleus). ASC, adipocyte stem cells; APC, adipocyte progenitor cells; Mono, monocytes; DCs, dendritic cells; ILCs, innate lymphoid cells. d, Cell-type proportions (for the cell types in c) in the combined cohort, mean per group, and for each sample (single nucleus). e, Correlations between cell types and clinical traits (Pearson, LN and OB samples only, single nucleus). Illustration in a created using BioRender (Scott, W., https://BioRender.com/rtmnzaj; 2025).
a, Graphical representation of the primary study cohort (left; single-nucleus analyses in n = 25 obese (OB) people before and after WL and n = 24 lean (LN) people, with spatial analyses in n = 4 people per group) and AT anatomical location (right). b, Clinical characteristics of the primary cohort (n = 24 LN and 25 paired OB–WL donors). Boxplot, median interquartile range minimum and maximum. BMI, body mass index (kg m–2); F insulin, fasting insulin (mIU L–1); HbA1c, haemoglobin A1c (%); HDL, high-density lipoprotein cholesterol (mM); DBP, diastolic blood pressure (mm Hg). c, Uniform manifold approximation and projection (UMAP) of 145,452 human AT cells (n = 74 samples of the primary cohort and n = 13 samples of the Emont published cohort11, single nucleus). ASC, adipocyte stem cells; APC, adipocyte progenitor cells; Mono, monocytes; DCs, dendritic cells; ILCs, innate lymphoid cells. d, Cell-type proportions (for the cell types in c) in the combined cohort, mean per group, and for each sample (single nucleus). e, Correlations between cell types and clinical traits (Pearson, LN and OB samples only, single nucleus). Illustration in a created using BioRender (Scott, W., https://BioRender.com/rtmnzaj; 2025).
Conclusions
This study marks a prominent leap in scientific understanding of adipose tissue biology, highlighting that while weight loss can partially and selectively reverse the cellular damage inflicted by obesity (reducing inflammation, senescence in specific lineages, and tissue stress), some immune and metabolic programs retain obesity-associated signatures, potentially explaining the stubborn tendency for weight to return.
The findings highlight fat as a complex, adaptive organ exhibiting cell-type-specific memory, suggesting that future interventions targeting unresolved senescence (e.g., in macrophages) or niche-specific stress signals (e.g., THBS1/NAMPT) may improve long-term outcomes.