Leica Microsystems’ optical coherence tomography (OCT) division Bioptigen received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its EnFocus intrasurgical OCT system.
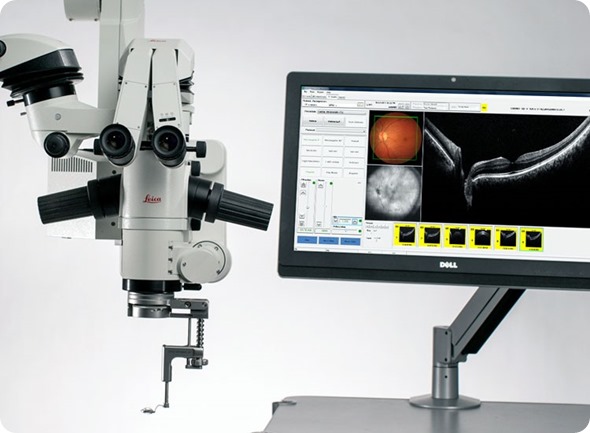
EnFocus is an upgrade solution for new and existing ophthalmic surgical microscopes, extending their capability. EnFocus allows visualization of ocular tissue microstructure during ophthalmic surgery in high-resolution, thereby providing both anterior and posterior surgeons with subsurface insight while operating.
“The EnFocus intrasurgical OCT system provides excellent optical imaging performance for the surgeon employing a modular design which enables sharing between multiple microscopes in an operating theater.”
“It cleverly adds an important capability to ophthalmic microscope platforms. The device is an innovative advancement in ophthalmic imaging that Leica customers called upon us to deliver, similar to the newly introduced Proveo ophthalmic microscope platform.”
Markus Lusser, President of Leica Microsystems.
“We are very excited to receive clearance to introduce the EnFocus intrasurgical OCT system, which provides ophthalmic surgeons with highly resolved, real-time, deep and wide-field images for use during ophthalmic surgery”, added Eric Buckland, CEO of Bioptigen. “This product introduction builds upon Bioptigen’s 10-year history of delivering advanced OCT imaging tools to the ophthalmic healthcare community”.
The EnFocus imaging systems, while mounted to surgical microscopes, are intended for use with patient populations from pediatric to adults. The systems acquire, process, and display depth-resolved images of ocular tissue using Spectral Domain Optical Coherence Tomography (SD-OCT), and are compatible with common retina fundus viewing systems.