Nov 29 2018
The Native Antigen Company has launched a series of ground-breaking immunoassays for Zika virus, offering researchers in academia, public health and drug discovery the capability to work with unprecedented levels of specificity and sensitivity. Unlike previous methods, the new assays have little or no cross-reactivity with other flaviviruses, including Dengue, West Nile, Yellow Fever, Japanese Encephalitis, Tick-Borne Encephalitis and Usutu virus. The Zika virus poses a significant global risk and there is currently no vaccine or specific treatment available for those infected. Sensitive and specific diagnostic assays are crucial for enabling effective research, epidemiology and disease management, as well as the development of new vaccines and therapeutics.
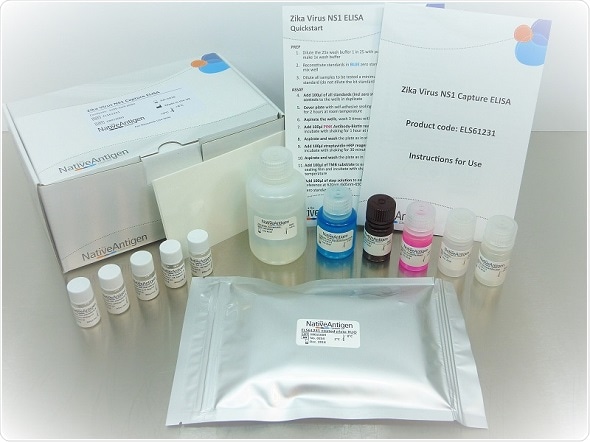
The Native Antigen Company Zika Virus NS1 ELISA assay
The Native Antigen Company Zika Virus NS1 ELISA assay is a highly sensitive and specific assay suitable for the quantitative detection of Zika Virus NS1 in a range of biological samples, with no cross-reactivity with other flaviviruses. Present in human serum during the early stages of infection, the NS1 protein provides an early marker of viral replication following infection. This ELISA assay can detect NS1 antigen at levels as low as 5 pg/ml and enables researchers to measure the level of Zika NS1 protein in infected patients, offering value in epidemiology studies.
The Native Antigen Company Zika Virus IgG/IgM/IgA ELISA assay is designed for the detection of Zika-specific antibodies in human serum. It is minimally cross-reactive with antibodies to Dengue virus and so can be used to distinguish human anti-Zika antibodies from other flavivirus and infectious disease antibodies. Previous assays have been time-consuming, cumbersome and have been unable to reliably distinguish between Zika and Dengue. However, the Zika Virus ELISA has an assay time of only 2 hours, with specificity and sensitivity both in excess of 90%, even in regions endemic with Dengue. Due to the novel assay format, the test may also be used in any species, making it of particular value for studying animal models of Zika infection.
In an independent assessment of the Zika Virus IgG/IgM/IgA ELISA kit, external researchers reported a sensitivity of 90.3% and specificity of 92.1%. This compared favourably with direct testing against a market-leading IgG assay that exhibited only 69.4% specificity.
Dr Andy Lane, Commercial Director at The Native Antigen Company said:
The launch of these next generation assays represents a significant leap forward for Zika virus detection and monitoring, which to date has always been hampered by Zika’s cross-reactivity with dengue. The impact of this exciting development will be seen across academia, public health and the pharmaceutical industry.”
In addition to the kits, The Native Antigen Company also offers an extensive range of Zika virus antigens, both expressed as recombinant proteins in a mammalian expression system and as native viral preparations. Further products include NS1 proteins (from both the prototypic Uganda strain and from the Suriname strain responsible for the major outbreak in 2016) and Zika Virus-like particles (VLPs).