In a collaborative study published in Blood Advances, a research group led by Beate Heissig at Juntendo University and Yousef Salama at An Najah University report a strategy to overcome drug resistance developed by blood cancer cells.
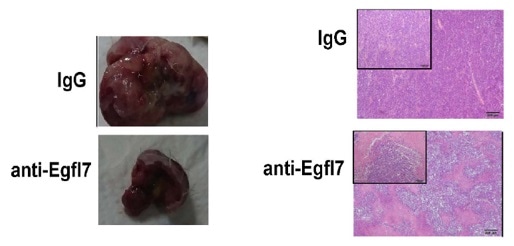
(Left): Blocking EGFL7 prevents multiple myeloma cancer tumors from growing. Researchers at Juntendo University studied the growth factor EGLF7 for its role in myeloma cell growth in mice. Mice treated with antibodies that target EGFL7 (anti-Egfl7) had smaller tumors than those treated with control antibodies (IgG; left images). (Right): Tumor tissues from control tumors show only tumor cells (dark blue stained), while in tumor tissues from antibody-treated animals vast areas of dead tumor tissues (necrotic tissues) can be found. Image by Salama et al. originally published by Blood Advances.
Multiple myeloma is an aggressive cancer affecting a specific type of white blood cells known as plasma cells. The primary challenge faced by oncologists in treating multiple myeloma is the tendency of the disease to recur after treatment or stop responding to treatment altogether. A team spanning across Juntendo University, the University of Tokyo, and An-Najah National University has now shown how a triad of proteins within cancerous plasma cells helps them grow uncontrollably and bypass the effects of cancer drugs.
The researchers first isolated affected blood cells from patients at various stages of cancer. Incidentally, the cells of all these patients had one thing in common—an increased presence of Egfl7, a protein that promotes cell growth. As expected, when Egfl7 levels were reduced within the cells, the cells started dying. Egfl7 was thus instrumental in helping the cancer cells grow. The proliferation of cancer cells requires Itgb3, a protein that coincidentally also acts as a partner of Egfl7. When the blood cells were further analyzed, indeed it was found that the two proteins latched onto each other and led to the survival of plasma cells.
The plasma cells of patients that do not respond to drugs hide in the blood-making niche, the bone marrow. But for a chemotherapeutic drug to work, cells need to proliferate. This prompted the group to investigate whether Egfl7 and Itgb3 were the culprits driving drug resistance. To assess this theory, cancerous plasma cells were treated with the anti-cancer drug bortezomib. Cells treated with higher doses of bortezomib did in fact have excessive levels of Egfl7 and Igtb3, making them more prone to hide out in the bone marrow and evade chemotherapy. This was accompanied by enhanced survival, proving that cancer had become insensitive to bortezomib. However, upon blocking Egfl7 and Igtb3 the cells began responding to bortezomib again. Lastly, it was also noted that the teaming up of Egfl7 and Igtb3 led to the activation of a third perpetrator—Klf2. Klf2, in turn, further enhanced Egfl7 levels, setting into motion a vicious cycle that kept tumor cells alive.
“Chemotherapeutic drugs are more effective when combined with drugs against Egfl7, thereby interrupting the vicious cycle,” said the researchers. What’s more, Egfl7-blockers, when used in combination with chemotherapeutic drugs, can enhance the effects of the latter especially in patients that are resistant to or do not respond well to chemotherapy. “In the present study, we provide functional evidence that the Egfl7-Itgb3-Klf2 axis regulates [multiple myeloma] cell growth and contributes to [bortezomib] resistance,” conclude the researchers. Blocking Egfl7 and its partners could be a promising strategy in not only preventing the growth of cancerous blood cells but also preventing resistance to existing chemotherapies.
Source:
Journal reference:
Salama, Y., et al. (2020) The Egfl7-Itgb3-Klf2 axis enhances myeloma cell survival and contributes to drug resistance. Blood Advances. doi.org/10.1182/bloodadvances.2019001002.