New evidence urges caution with TMP-SMX antibiotics in early pregnancy, highlighting safer options for treating urinary tract infections.
 Study: First-Trimester Antibiotic Use for Urinary Tract Infection and Risk of Congenital Malformations. Image Credit: HenadziPechan / Shutterstock
Study: First-Trimester Antibiotic Use for Urinary Tract Infection and Risk of Congenital Malformations. Image Credit: HenadziPechan / Shutterstock
In a recent study published in JAMA Network Open, researchers evaluated the risk of congenital malformations following antibiotic treatment for urinary tract infection (UTI) during the first trimester of pregnancy.
UTIs, such as acute cystitis and asymptomatic bacteriuria (ASB), are common in pregnancy and are associated with adverse outcomes, including low birth weight, preterm birth, maternal sepsis, and pyelonephritis. Routine ASB screening is recommended at the initial prenatal visit, often leading to antibiotic therapy in the first trimester.
However, studies have raised concerns about higher risks of congenital malformations associated with two antibiotics used for UTI treatment: trimethoprim-sulfamethoxazole (TMP-SMX) and nitrofurantoin, albeit these studies have significant methodological limitations. Therefore, evidence on the safety of antibiotics in pregnancy is necessary to guide clinical practice.
About the study
In the present study, researchers investigated whether TMP-SMX, fluoroquinolones, and nitrofurantoin were associated with congenital malformations compared to other antibiotics used for UTI treatment in the United States (US). First, pregnant individuals aged 15–49 receiving antibiotics for UTI in their first trimester and their live-born infants were identified from a commercially insured population between 2006 and 2022.
Individuals receiving more than one UTI-related antibiotic, those with a spinal cord injury, immunosuppression, chromosomal abnormalities, and pregnant women exposed to a teratogenic medication in the first trimester were excluded. Outpatient pharmacy claims were used to identify antibiotics dispensed for UTI treatment, which included TMP-SMX, nitrofurantoin, β-lactams, and fluoroquinolones (ofloxacin, ciprofloxacin, and levofloxacin).
β-lactams were used as the reference, as these agents are deemed safe in pregnancy. The study’s outcomes were congenital malformations that were identified using validated claims-based algorithms adapted from Kharbanda et al. Log-binomial regression models assessed the association between antibiotics and malformation risk. Potential confounders included demographic characteristics, concomitant medication, comorbidities, and healthcare utilization measures.
Further, numerous sensitivity analyses were performed to evaluate the robustness of findings. These included restricting the reference group to cephalexin or amoxicillin, using an alternative outcome definition, and restricting the cohort to symptomatic UTI. The team also explored gestational timing effects by narrowing exposure windows to critical organogenesis periods (e.g., 4-9 weeks for cardiac defects). Finally, the team examined associations between antibiotics and the risk of cardiac malformations and any malformations in a larger cohort of pregnant individuals receiving UTI-related antibiotics for any indication.
Findings
The study included 71,604 pregnancies, with 59.2% exposed to nitrofurantoin, 30.8% exposed to β-lactams, 5.1% exposed to fluoroquinolones, and 4.9% exposed to TMP-SMX for UTI treatment in their first trimester. Median gestational age at antibiotic exposure differed significantly: TMP-SMX and fluoroquinolones were prescribed earlier (26 and 18 days post-LMP, respectively) than β-lactams or nitrofurantoin (63 and 62 days). In total, 1,518 infants were identified with congenital malformations. The absolute, unadjusted risk of any congenital malformation was 19.8, 21.2, 23.5, and 26.9 per 1,000 infants for β-lactams, nitrofurantoin, fluoroquinolones, and TMP-SMX, respectively. Estimates were similar for cardiac malformations.
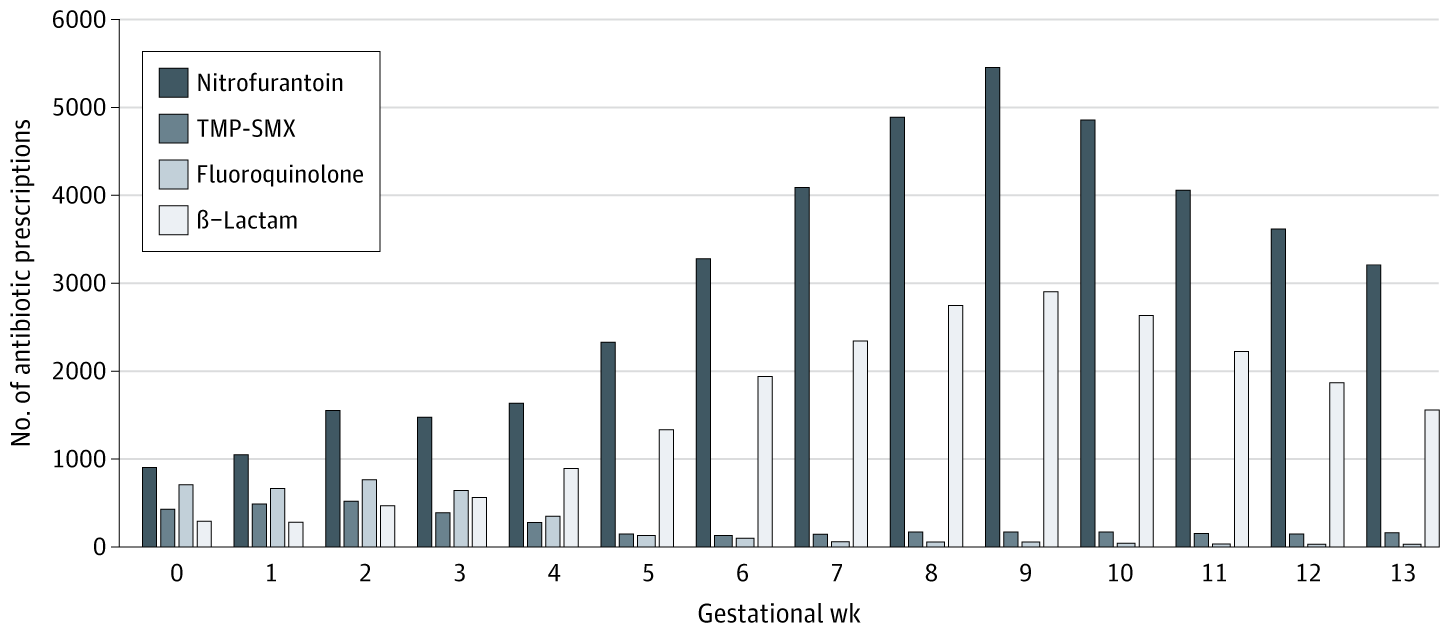 The median (IQR) gestational age at index for symptomatic UTI was 6 (3-9) weeks compared with 9 (7-11) weeks for asymptomatic bacteriuria. TMP-SMX indicates trimethoprim-sulfamethoxazole.
The median (IQR) gestational age at index for symptomatic UTI was 6 (3-9) weeks compared with 9 (7-11) weeks for asymptomatic bacteriuria. TMP-SMX indicates trimethoprim-sulfamethoxazole.
Infants exposed to TMP-SMX had an increased risk of any malformation compared to those exposed to β-lactams (adjusted RR 1.35; 95% CI 1.04-1.75). The researchers estimated that one additional malformation would occur for every 145 pregnancies exposed to TMP-SMX. The malformation risk was similar for infants exposed to fluoroquinolones or nitrofurantoin compared to those exposed to β-lactams.
In organ-specific malformation analyses, there was a similar risk for cardiac malformations for β-lactam- and TMP-SMX-exposed pregnancies, but TMP-SMX was associated with increased relative risk for orofacial/respiratory malformations (RR 2.89; 95% CI 1.31-6.41) and specifically for severe cardiac defects (RR 2.09), other cardiac defects (RR 1.52), and cleft lip/palate (RR 3.23). However, risk difference estimates for these specific malformations included the null value, indicating uncertainty in absolute risk.
In sensitivity analyses:
- Alternative outcome definitions and reference groups yielded consistent results.
- Restricting to symptomatic UTIs (excluding ASB) did not meaningfully change effect estimates.
- Analyses accounting for gestational timing showed TMP-SMX risk persisted but became less precise when narrowed to organogenesis windows.
Finally, the larger cohort included 256,686 pregnant individuals receiving UTI antibiotics for any indication. This alternative analysis was specifically designed to address potential confounding by indication. In this cohort, the risk of any malformation was higher among pregnancies exposed to TMP-SMX specifically for UTI than for other indications. The risk did not vary by indication for other antibiotics. Further, the risk for cardiac malformations or any malformations was similar for infants exposed to fluoroquinolones, TMP-SMX, or nitrofurantoin compared to those exposed to β-lactams. However, analyses for fluoroquinolones were limited by smaller sample sizes (n=3,663), resulting in wider confidence intervals and less precise estimates.
Conclusions
Together, TMP-SMX exposure for UTI treatment in the first trimester was associated with a higher risk of any congenital malformation. It was also associated with an increased relative risk (though uncertain absolute risk) for severe cardiac, cleft lip, cleft palate, and other cardiac malformations compared to β-lactams.
Notably, the risk of malformation was not higher with nitrofurantoin compared to β-lactams. The findings were consistent across sensitivity analyses, although fluoroquinolone comparisons should be interpreted with caution due to limited statistical power.
The study’s limitations include its non-randomized design, which may subject the results to residual confounding, possible outcome misclassification, exposure misclassification, selection bias due to the restriction to live births, and non-generalizability of the results to uninsured or Medicaid-insured populations.
Overall, the results support current recommendations for caution in using TMP-SMX in the first trimester but do not support limiting nitrofurantoin use.