The current COVID-19 pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and has become a significant public health crisis around the world. It is a multisystemic illness, though it appears to be most recognizable as severe pneumonia leading to acute respiratory distress syndrome (ARDS) in a significant minority of cases.
The fact remains that the infection is asymptomatic and, therefore, unrecognizable in most cases, allowing its extensive and rapid spread. There is a great need to identify biomarkers capable of predicting the severity of the disease early on, so as to help manage such patients appropriately. Moreover, the mechanism of disease is also far from clear.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Multisystem Disease
Prior research shows that besides the lungs, COVID-19 also affects the blood, heart, kidneys, liver, and brain. An uncontrolled immune response is also thought to be a contributor to critical COVID-19 disease, manifesting in some pediatric patients as multisystem inflammatory syndrome.
The current study was aimed at exploring the use of cell-free DNA (cfDNA) in circulation in an attempt to find tests that can quantify the injury occurring to multiple tissues simultaneously. One use of such tests will be to monitor the occurrence of injury to the cells, tissues, and organs of a COVID-19 patient. The second is to evaluate the severity of disease and predict its outcomes. And thirdly, they can help uncover the involvement of multiple organs in this disease.
What is cfDNA?
Non-invasive prenatal testing was made possible for the first time after finding that cfDNA could be used to detect genetic anomalies in the fetus. These are short DNA pieces circulating after the destruction of their containing cells. Again, after solid-organ transplants, cfDNA from the donor marks the occurrence of early rejection.
More recently, cfDNA is being tested by epigenetic techniques to identify its tissue of origin. The present research centers on this hypothesis, aiming to identify the injured tissues/organs in COVID-19.
The study: cfDNA and Tissues of Origin
The researchers examined 52 blood samples from 5 adults with COVID-19, collected over 14 days, along with six samples from patients with other respiratory infections such as influenza B, respiratory syncytial virus, and the endemic coronaviruses. They found that cfDNA from the lung was increased in COVID-19 compared to other respiratory infections, as well as liver and blood cell precursors.
They then tested 52 more samples from 28 COVID-19 patients enrolled in a clinical trial, collected at days 1, 5, and 15 from the time of enrolment as long as they were still in hospital at the date of collection. They found that lung cfDNA was higher than the highest average concentration in over 60% of these patients, and also higher liver-derived cfDNA and erythroblast-derived cfDNA. Concerning the time frame, they found that the tissue injury slowly recovered over time, with a slow increase in the relative contribution of erythroblast-derived cfDNA over time.
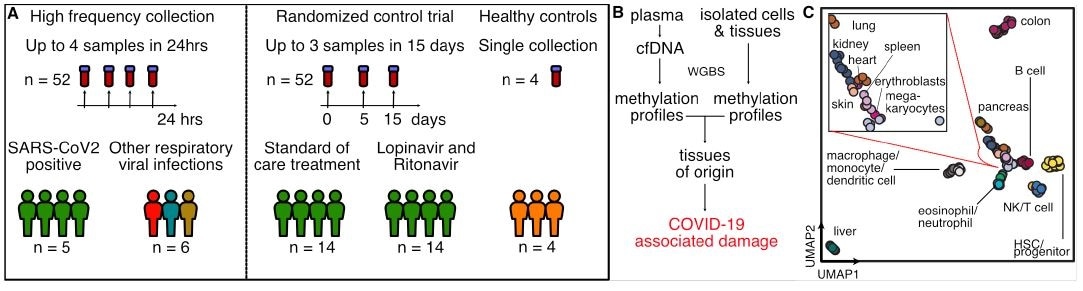
Study design. A) Two independent cohorts were used in our study: First, a high frequency collection cohort with 5 SARS-CoV-2 patients (n = 52 samples) and 6 SARS-CoV-2 negative, RNA-virus positive patients (n = 6 samples). Second, a randomized control trial of 28 SARS-CoV-2 patients with plasma at serial time points (n = 52 samples). 4 healthy individuals volunteered plasma for cell-free DNA analysis. B) Experimental workflow. cfDNA is extracted from plasma and whole-genome bisulfite sequencing is performed. In parallel, methylation profiles of cell and tissue genomes are obtained from publicly-available databases. cfDNA methylation profiles are compared to those of cell and tissue references to infer relative contributions of tissues to the cfDNA mixtures. C) UMAP of differentially methylated regions for isolated cell and tissue types used as a reference.
cfDNA Reflects Injury and Tissue of Origin
The researchers found that in two different cohorts of COVID-19 patients, there were signs of injury to the lung, liver, and kidney. They also found that cfDNA from red blood cell precursors increased both relatively and absolutely in these patients compared to those with other RNA virus infections and healthy controls.
Kidney cfDNA was higher in COVID-19 patients who died, at 1.5% vs. 0.5% in those who survived, and 0.005% in healthy controls.
An increase in the relative proportion of erythroblast-derived cfDNA showed that at any point, it predicted mortality of the hospitalized patient. At a score of 9, the patients had higher erythroblast cfDNA than when the score was 7-8, though all patients had significantly increased erythroblast cfDNA.
This is thought to be due to increased red cell turnover and production following COVID-10-induced hemolysis, which in turn is signaled by the anemia common in these patients. In fact, this cfDNA corresponds to clinical measures of anemia. The mechanisms by which anemia is caused include hyperinflammation and cytokine storm, phagocytosis of red cells secondary to inflammation, and the formation of microthrombi, which consumes red cells.
The researchers support another putative mechanism, however, namely, dysregulated red cell production secondary to virus-ACE2 binding, which acts via angiotensin II. Direct or indirect injury to the red cell precursors is another proposed source of cfDNA. Further research into the circulating levels of reticulocytes and bone marrow evaluation will be required to test these possibilities.
Liver cfDNA correlates with liver enzymes. In agreement with another paper, the study also found that lactate dehydrogenase (LDH), known to be a marker of tissue damage and of red cell breakdown, is a strong predictor of COVID-19 outcome, correlating well with erythroblast cfDNA. The use of tissue-specific cfDNA may help to detect the damaged tissue of which LDH serves as a marker.
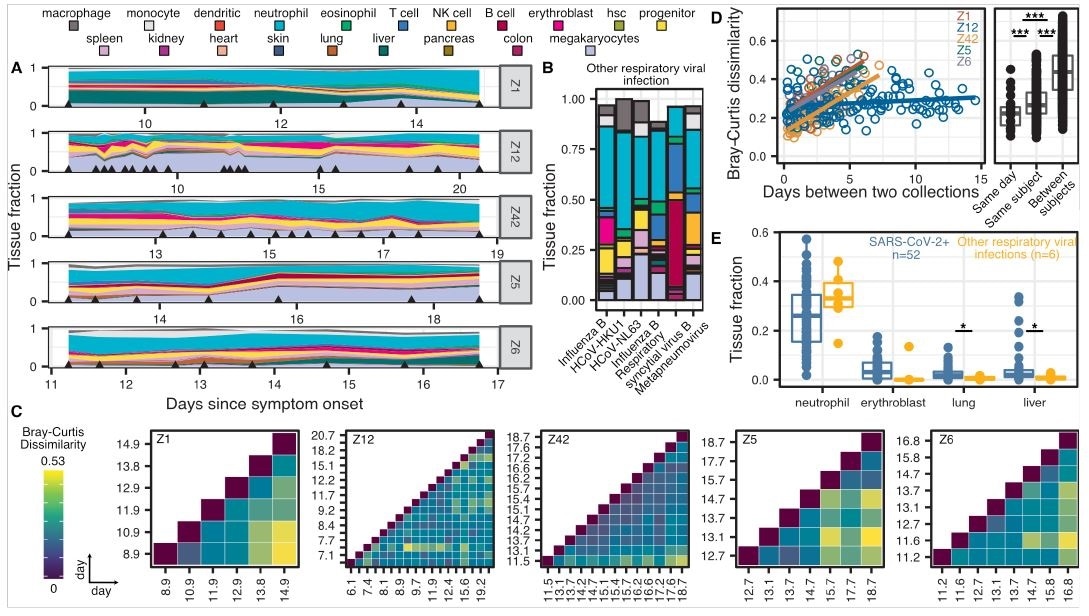
High frequency sample collection cohort at UCSF. A-B) Patient-specific relative tissue contributions for SARS-CoV-2 patients (A) and other RNA-virus infection patients (B). Triangles indicate sampling times. C) Heatmaps of Bray-Curtis dissimilarity. D) Scatterplot of patientspecific Bray-Curtis dissimilarity (left) and boxplot of Bray-Curtis dissimilarity between cfDNA tissue proportions from samples collected from either the same day (within 24 hours), the same person (but not within 24 hours), or from all patients (right). E) Comparison of tissue fraction of four cell and tissue types (neutrophil, erythroblast, lung and liver) between SARS-CoV-2 positive patients and other RNA-virus positive patients. * : p-value < 0.05; ** : p-value < 0.01; *** : p-value < 0.001 (p-values calculated using a Wilcoxon test)
Prognosis Linked to Total cfDNA
The study observes, “Clinical score of 7 or greater (indicating the need for admission to the intensive care unit and invasive mechanical ventilation), was associated with a sharp increase in the total burden of cfDNA.” The latter increased tenfold frp, 1.5 g/μL in patients with scores between 4-6 and 7-9.
The researchers found that the total amount of cfDNA was comparable to the WHO ordinal scale for progressive disease. As the amount of cfDNA increased, so did the number of admissions to the intensive care unit (ICU) and the number of patients who needed mechanical ventilation.
Interestingly, there was no significant difference in the levels of cfDNA in the serum of patients treated with lopinavir/ritonavir, supporting earlier trials that did not provide definitive proof of the efficacy of this treatment above standard care.
Implications and Conclusion
The study sums up,” We find significant support for the utility of cfDNA profiling as a prognostic tool for the early detection and monitoring of cell and tissue injury associated with COVID-19.” This minimally invasive test not only allows a more detailed picture of the clinical severity of the disease at the time of presentation but also provides evidence of its correlation as a reliable biomarker of severity in clinical trials of new drugs proposed for the treatment of COVID-19.
Secondly, they narrow down a specific aspect, namely, the total cfDNA, which can be measured cheaply within an hour, for comparison of patient outcomes in clinical trials as well as for determining patient management in current care.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Cheng, A. P. et al. (2020). Cell-Free DNA in Blood Reveals Significant Cell, Tissue and Organ Specific injury and Predicts COVID-19 Severity. medRxiv preprint. doi: https://doi.org/10.1101/2020.07.27.20163188. https://www.medrxiv.org/content/10.1101/2020.07.27.20163188v1
- Peer reviewed and published scientific report.
Cheng, Alexandre Pellan, Matthew Pellan Cheng, Wei Gu, Joan Sesing Lenz, Elaine Hsu, Erwin Schurr, Guillaume Bourque, et al. 2021. “Cell-Free DNA Tissues of Origin by Methylation Profiling Reveals Significant Cell, Tissue, and Organ-Specific Injury Related to COVID-19 Severity.” Med 2 (4): 411-422.e5. https://doi.org/10.1016/j.medj.2021.01.001. https://www.cell.com/med/fulltext/S2666-6340(21)00031-3.