A group of Chinese researchers recently conducted a phase 2 clinical trial to further evaluate the immunogenicity and safety of SARS-CoV-2 inactivated vaccine known as CoronaVac. The results are currently available on the medRxiv* preprint server and demonstrate that the vaccine is well tolerated and without any notable dose-related safety concerns, which opens the door for phase 3 clinical trial.
The coronavirus disease (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), continues to affect the health and change lifestyles of people around the world. Many argue that there will be no return to normalcy without an effective vaccine.
As a result, in August 2020, there were more than 231 vaccine candidates in development, and at least 24 of them progressed to clinical trials. Currently, there are 18 of them in phase 1-2, while 6 are beginning with phase 3.
The inactivated SARS-CoV-2 vaccine developed by Sinovac Life Sciences Co. Ltd. from China (also known as CoronaVac) has been shown to be safe in preclinical trials. It could also induce SARS-CoV-2 specific neutralizing antibodies in rats, mice, and nonhuman primates.
And since no safety concerns have been found in phase 1 clinical trial with CoronaVac, a research group led by Dr. Yanjun Zhang from the Department of Microbiology, Zhejiang Provincial Center for Disease Control and Prevention in Hangzhou (China) conducted a phase 2 clinical trial.
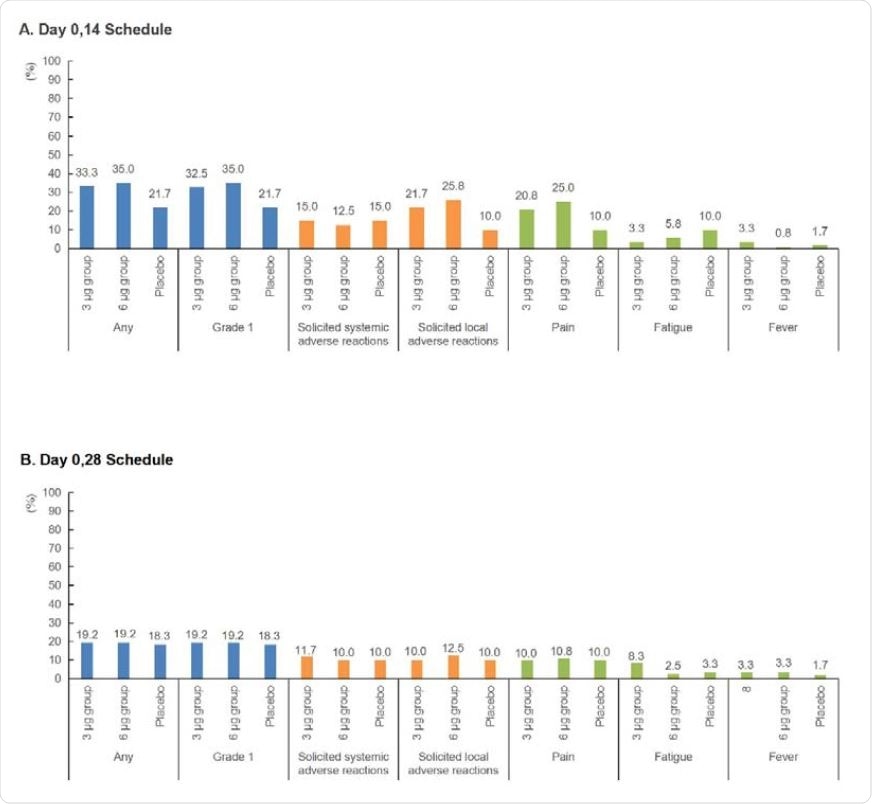
Incidence rates of adverse reactions among different groups in phase 2. (A) The incidence rates of adverse reactions among different groups with a Day 0,14 schedule. (B) The incidence rates of adverse reactions among different groups with a Day 0,28 schedule.
Trial design and oversight
The study was designed as a randomized, double-blind, placebo-controlled trial in order to appraise the optimal dosage, safety, and immunogenicity of the CoronaVac vaccine. A total of 600 healthy adults aged 18-59 years were included in the study.
Participants were randomly assigned into three groups (i.e., in a ratio of 2:2:1) to receive two injections of the tested vaccine at a dose of 3 μg/0.5 mL or 6 μg/0.5mL, or placebo on Day 0,14 schedule or Day 0,28 schedule.
For safety evaluation, all solicited and unsolicited adverse events were collected after each vaccination within 7 days and 28 days, respectively. Immediate adverse events were also assessed on-site for at least 30 minutes after each vaccine administration.
To evaluate the immune response, blood samples were collected from each participant at different time points. The ability of the antibodies to bind the receptor-binding domain of SARS-CoV-2 was assessed by enzyme-linked immunosorbent assay while neutralizing antibody titers were measured with the use of a modified cytopathogenic effect assay.
Favorable safety and immunogenicity profile
"This trial demonstrated that the 2 doses of different dosage of CoronaVac were well tolerated and immunogenic in healthy adults aged 18-59 years", say study authors. "The incidence rates of adverse reactions in the 6 μg and 3 μg groups were comparable, indicating that there was no dose-related aggravating concern on safety", they add.
Moreover, there were no serious adverse events related to the vaccine, and most reported side effects were generally mild (with pain at the injection site as the most frequently reported symptom). In a nutshell, the safety profile of CoronaVac is similar to that observed in phase 1 clinical trial.
Conversely, immune responses that were elicited in phase 2 were superior to those recorded in phase 1, revealing seroconversion rates over 90%. Furthermore, after two-dose vaccination, immune responses induced by Day 0,28 schedule were above the value of Day 0,14 schedule – regardless of the vaccine dosage.
When antibody levels were compared between different age groups, it should be emphasized that the neutralizing antibody titers dropped significantly with increasing age. These results are highly consistent with epidemiological trends observed in patients with COVID-19.
Towards the phase 3 clinical trial
"Favorable safety and immunogenicity of CoronaVac was demonstrated on both schedules and both dosages, which support the conduction of phase 3 trial with optimum schedule/dosage per different scenarios", highlight study authors in their medRxiv paper.
Compared with other COVID-19 vaccine contenders, the incidence rate of fever was relatively low in this clinical trial, further indicating that CoronaVac was actually well tolerated.
Hence, the next priority for the researchers is to evaluate the protective efficacy of the 3 μg dosage under Day 0,14 schedule. Likewise, the researchers state in the paper that day 0,28 schedule with 3 μg vaccine will also be adopted in the future phase 3 clinical trial. These results are eagerly expected.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Zhang, Y.J. et al. (2020). Immunogenicity and Safety of a SARS-CoV-2 Inactivated Vaccine in Healthy Adults Aged 18-59 years: Report of the Randomized, Double-blind, and Placebo-controlled Phase 2 Clinical Trial. medRxiv. https://www.medrxiv.org/content/10.1101/2020.07.31.20161216v1
- Peer reviewed and published scientific report.
Zhang, Y., Zeng, G., Pan, H., Li, C., Hu, Y., Chu, K., Han, W., Chen, Z., Tang, R., Yin, W., Chen, X., Hu, Y., Liu, X., Jiang, C., Li, J., Yang, M., Song, Y., Wang, X., Gao, Q., & Zhu, F. (2020). Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. The Lancet Infectious Diseases, 0(0). https://doi.org/10.1016/S1473-3099(20)30843-4, https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30843-4/fulltext