A new study by scientists in the Netherlands and published on the preprint server bioRxiv* in August 2020 shows that the severe acute respiratory coronavirus-2 (SARS-CoV-2) can infect rabbits, which opens the door for possible circulation in rabbit farms and another potential source of animal to human SARS-CoV-2 infection. This finding calls for urgent research on the prevalence of the virus in farmed rabbits.

Study: Susceptibility of rabbits to SARS-CoV-2. Image Credit: TY Lim / Shutterstock
Even as public health authorities and scientists team up to contain the pandemic, the SARS-CoV-2 transmission continues. Almost from the beginning, one big concern has been that the virus might infect secondary reservoir hosts, propagate in them, and re-infect humans again.
Slowing the transmission of the virus requires knowledge of the many routes of spread between humans as well as between humans and other species. So far, the virus has been found to infect cats, ferrets, non-human primates, hamsters, and even dogs to some extent, while it can spread from ferrets, cats, and hamsters to other animals via airborne transmission.
Pet dogs and cats not only pick up the virus but also transmit it readily. Farmed mink in many Dutch farms also show the same susceptibility to infection, with workers on these farms also found to be infected with viruses that are phylogenetically very similar to those found in the animals.
These findings show the urgency of containing viral transmission not only among humans but also through these spillover events, among animals who are in close proximity with humans, either as pets or as farmed animals. The latter is of particular interest, because of the crowding of animals in many such farms, which encourages rapid spread. Since rabbits are among the most commonly farmed animals worldwide, the current study focused on identifying how far these creatures are susceptible to the virus.
Does Rabbit ACE2 Support SARS-CoV-2 Entry?
One key determinant for the host range of the virus is the angiotensin-converting enzyme 2 (ACE2), which is the viral receptor on the host cell. Contact residues of human and rabbit ACE2 critical for binding S are relatively well conserved.
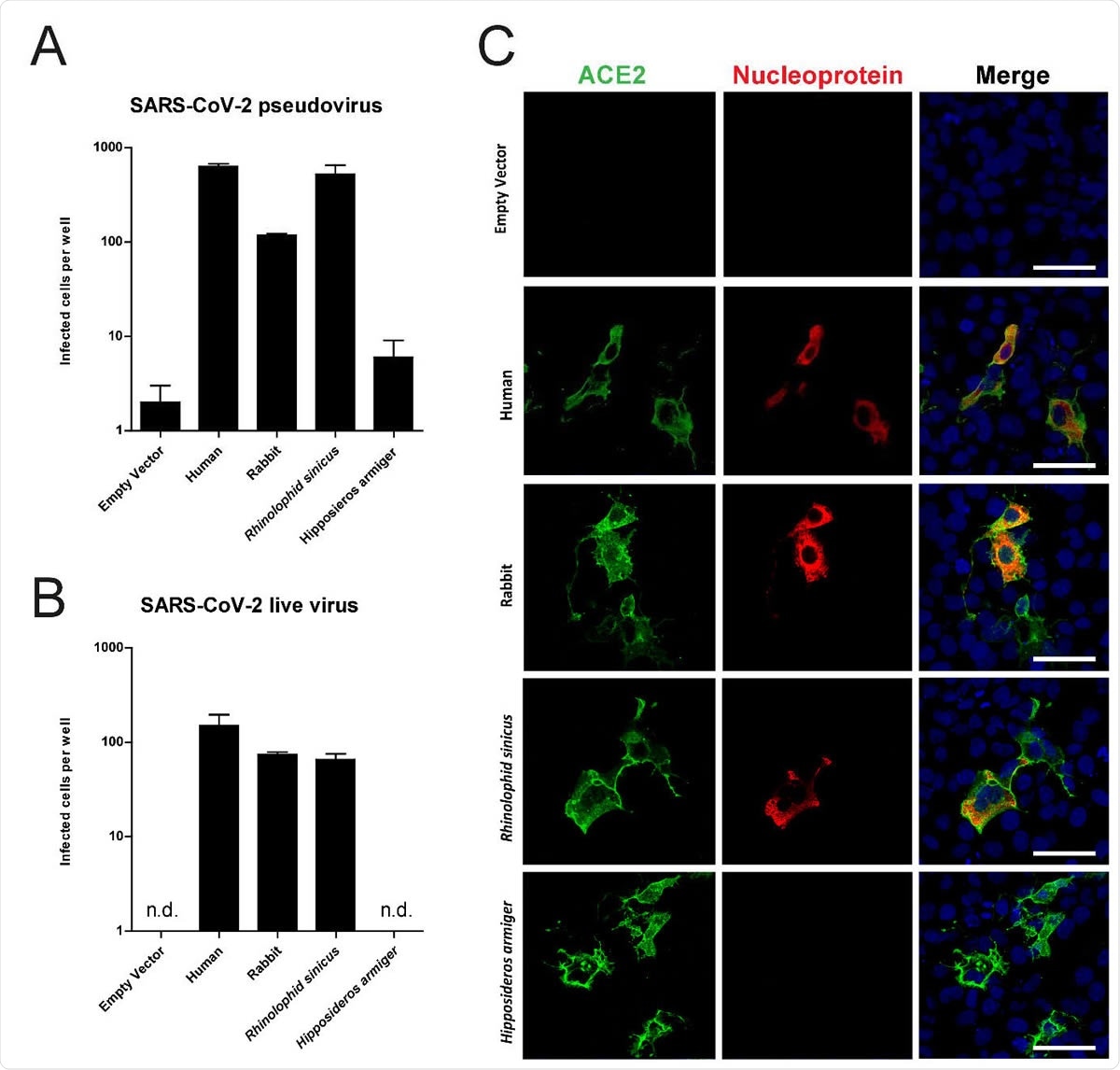
Rabbit ACE2 mediated SARS-CoV-2 infection. SARS-CoV-2 pseudovirus (A) and authentic infection of Cos-7 cells expressing ACE2 of various species. Infectivity was quantified by staining live v with anti-SARS-CoV nucleocapsid and scanning live virus and pseudovirus infected cells. (C) Confocal im ACE2 mediated live virus infection; cells were stained using anti-human ACE2 in green, anti-SA nucleocapsid in red and TO-PRO3 in blue to stain nuclei. Scale indicates 50μm.
The researchers in the current study overexpressed ACE2 from various species (humans, rabbits, and the Chinese horseshoe bat) in a cultured cell line, which naturally lacks receptors. After this, they exposed this cell line to infection with both SARS-CoV-2 pseudovirus and the wildtype virus. As a control, they also used ACE2 from another bat species, which does not serve as a receptor for this virus. They found that when rabbit ACE2 was transfected, the cells became susceptible to SARS-CoV-2.
Upper Respiratory Infection with SARS-CoV-2
The next step was to inoculate three rabbits with 106 infectious doses (TC1D50) and followed them for signs of infection for three weeks. They found none. However, they were able to recover viral RNA from the nose for this period, from the throat for up to 14 days, and in the rectum for up to 9 days. The mean period of shedding in these three locations was 15, 11, and 5 days respectively.
Infectious virus particles were present in the nasal secretions for up to one week with peak shedding on day 2 and day 7. In the throat, this occurred only on the first day after inoculation and in only one animal. Infectious virus was not recovered in rectal swabs.
Seroconversion occurred in all animals by day 21, and the plaque reduction neutralization test (PRNT50) showed titers of 1:40, 1:320, and 1:640.
Relative Sparing of Lungs
They also inoculated three groups of three animals each with 104, 105 or 106 TCID50, and examined swabs from each animal for four days, finally sacrificing them. This confirmed that all animals who received 106 TCID50 had detectable viral RNA in the nose and throat for up to 4 days, one animal also showing positivity in the rectal swabs on day 3. Lung tissue showed no viral RNA, however. Histological examination showed macrophage infiltration in the alveoli, scattered neutrophils, and mild scattered thickening of the septa around the terminal bronchioles. Other than mild necrotic changes, the most noticeable finding was severe bronchus-associated lymphoid tissue (BALT) proliferation, and sometimes enlargement of the tracheobronchial nodes as expected with mild lymphoid hyperplasia.
The olfactory epithelium showed similar lymphoplasmacytic infiltration, with a mild increase in the number and size of cells. All these findings show that the virus can grow and propagate in rabbits, though asymptomatically, with the peak viral titers being approximately 103 TCID50. The infectious virus was detected at up to 7 days from inoculation. However, a high inoculation dose (105 TCID or more) was necessary to establish transmission, which may indicate that ferrets and hamsters are more susceptible to the virus.
Limitations and Implications
On the other hand, the study used only young rabbits with robust immunity, and with no pre-existing disease, of a single breed. This may not be generalizable to other breeds or age groups of rabbits. Serologic testing may be needed, along with other surveillance studies, to evaluate to what extent farmed rabbits have been infected with this virus.
Secondly, the two-phase viral shedding of rabbits has also been seen to occur in African green monkeys and might be the result of early innate immune responses that kick in within days, partially clearing the virus. Adaptive responses may take longer, about a week, which allows a second peak to arise before it is cleared. Human studies show that a rise in neutralizing antibodies corresponds to a fall in viral shedding, but that viral RNA is shed longer than the infectious virus.
Thirdly, the detection of eosinophils in the nose and lungs of infected rabbits may indicate that T helper cells 2 (Th2) take part in the immune response. The study also shows that as in ferrets, the virus prefers to infect the upper respiratory tract while sparing the lungs.
The earlier detection of mink infection and the subsequent transmission to farm workers led to the mass elimination of infected mink. Similar steps may be necessary to limit viral spread from rabbits to humans on rabbit farms, the researchers suggest.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources