The coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by a range of symptoms including fever, cough, fatigue, and myalgia in the majority of cases and occasional headache and diarrhea. Of the 6% of patients admitted to ICU (Intensive Care Units), 2-8% of the cases turn fatal (943,203 deaths due to COVID-19 as of September 18, 2020)1. Severe COVID-19 infection progresses to acute respiratory distress syndrome (ARDS), with numerous patients developing life-threatening thrombotic complications. Lymphopenia is known to be a common feature in patients with COVID-19.
Postulating that the cellular and molecular levels of inflammation could represent a strong prognostic signature of the disease, Béhazine Combadiere et al. investigate the neutrophil subsets in severe and critical COVID-19 patients admitted to ICU and non-ICU departments. In a new bioRxiv* preprint research paper, they show that patients suffering from clinical severity also have high profiling of a specific neutrophil subset.
A massive influx of innate immune cells, namely neutrophils and monocytes, is associated with a cytokine storm (uncontrollable inflammatory response leading to viral sepsis, acute respiratory distress syndrome, respiratory failure, shock, organ failure, or death) – which is almost always associated with the disease severity. About 80% of the ICU patients had myelemia - a condition where large numbers of cells such as neutrophilic and eosinophilic myelocytes and nucleated red blood-corpuscles appear in the blood. These cells are typically found only in the bone marrow. The developed myelemia is found to be myelemia with CD10-CD64+ immature neutrophils. Investigating into its profile, the researchers found that two kinds of receptors were overexpressed in the ICU patients: lectin‐like oxidized low‐density lipoprotein receptor‐1 (LOX‐1) or the Interleukin-3 receptor alpha (CD123).
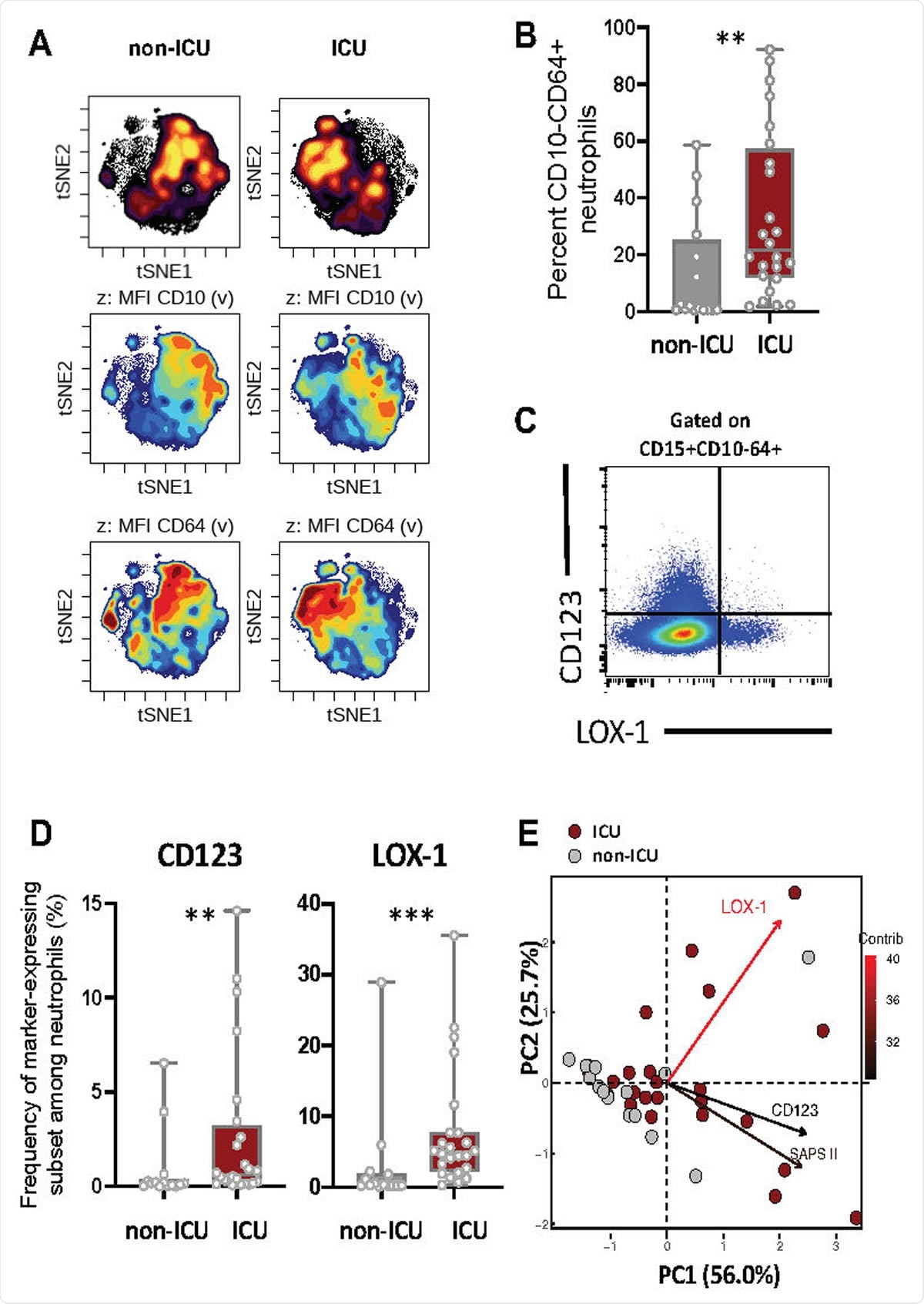
Severe COVID-19 patients displayed increased immature neutrophil subsets expressing CD123 or LOX-1. (A) viSNE analysis was performed on neutrophils from all samples with cells organized along t-SNE-1 and t-SNE-2 according to per-cell expression of CD15, CD10, CD64, LOX-1, CD123 and PD-L1. Cell density for the concatenated file of each patient’s group (ICU vs Non-ICU) is shown on a black to yellow heat scale. Neutrophils’ CD10, CD64 markers expression is presented on a rainbow heat scale in the t-SNE map of each group concatenated file. (B) Box plots representation (min to max distribution) of CD10- CD64+ neutrophil subset abundancy among total neutrophils of each group samples. (C) Representative expression of LOX1 and CD123 on CD10- CD64+ neutrophils. (D) Abundancy of CD10- CD64+ neutrophil expressing CD123 or LOX-1 in ICU and non-ICU patients’ groups. identify the median and min to max distribution. Nonparametric Mann-Whitney test was used to compare differences in cellular abundance of neutrophil subsets between groups, with significance defined by a p-value < 0.05: * for p < 0.05; ** for p < 0.01; *** for p < 0.001. (E) Principal component analysis (PCA) using LOX-1+, CD123+ CD10- CD64+ neutrophil abundancy and SAPS II variables on sample sizes: ICU=24 (dark red circles), non-ICU=14 (grey circles). Percent contribution (contrib) of each variable is indicated in color gradient black-red of the arrows.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The LOX-1-expressing immature neutrophils in patients correlated with high IL-1β, IL-6, IL-8, TNFα serum levels. The CD123-expressing immature neutrophils, also associated with the severity of the infection, correlated with a different cytokine profile: IL-18, IL-22, IFNγ secretion. These data suggest that these receptors may define a specific profile of severity associated with high levels of pro-inflammatory cytokines.
Patients with clinical severity were observed with a cytokine storm and intravascular coagulation. The LOX-1-expressing immature neutrophils were positively correlated with this observation. LOX-1 is a well-known marker of inflammation and neutrophils dysfunction in sepsis and cancers. Importantly, this research study shows that the high presence of these LOX-1-expressing immature neutrophils is associated with a high risk of severe thrombosis in patients.
Significance of this study
The researchers developed a multi-parametric neutrophil profiling strategy based on known neutrophil markers to distinguish COVID-19 phenotypes in critical and severe patients. Circulating immature neutrophils, with biomarkers CD123 or LOX-1, are found in these patients.
The researchers report for the first time the association between CD123 expression on immature neutrophils and high serum levels of IL-17, IL-22, and IFNγ.
While LOX-1 is a well-studied class E scavenger receptor acknowledged for its role in atherosclerosis, its role on neutrophils remained elusive. LOX-1 is barely detected during homeostasis on neutrophils; however, its expression is found on neutrophils in human cancer patients. Here, in COVID-19 patients, LOX-1 expression on immature neutrophils seems detrimental because it is associated with the secretion of several pro-inflammatory cytokines, such as IL-6, IL-1β, and TNFα, and with the severity of infection and thrombosis. The researchers also point out to determine whether thrombosis in COVID-19 patients results from functionally-diverted neutrophils expressing LOX-1 or from its expression on endothelial and smooth muscle cells.
The study involved a cohort of 38 COVID-19 patients admitted to either ICU or non-ICU departments, many of them with common past medical comorbidities such as hypertension, type 2 diabetes, and obesity. The authors recognize the need to confirm their observations using a larger cohort of patients. Owing to the significance of this study in developing therapeutic strategies, this calls for further detailed research.
Two potential, measurable biomarkers are identified in this study - significantly correlated with disease severity in general, and one of the markers, LOX-1, more particularly to thromboembolic events. The clinical significance of this study is that the patients can be tested for LOX-1-immature neutrophils counts at POC (point of care) testing. This enables quick medical decisions – distinguishing patients who may be at high risk for thrombosis complications. These patients may be helped with intensified anticoagulant therapy, improving their chances of a better and speedy recovery.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
LOX-1+ immature neutrophils predict severe COVID-19 patients at risk of thrombotic complications, Behazine Combadiere, Lucille Adam, Paul Quentric, Pierre Rosenbaum, Karim Dorgham, Olivia Bonduelle, Christophe Parizot, Delphine Sauce, Julien Mayaux, Charles-Edouard Luyt, Alexandre Boissonnas, Zahir Amoura, Valerie Pourcher, Makoto Miyara, Guy Gorochov, Amelie Guihot, Christophe Combadiere, bioRxiv 2020.09.15.293100; doi: https://doi.org/10.1101/2020.09.15.293100
- Peer reviewed and published scientific report.
Combadière, Behazine, Lucille Adam, Noëlline Guillou, Paul Quentric, Pierre Rosenbaum, Karim Dorgham, Olivia Bonduelle, et al. 2021. “LOX-1-Expressing Immature Neutrophils Identify Critically-Ill COVID-19 Patients at Risk of Thrombotic Complications.” Frontiers in Immunology 12 (September). https://doi.org/10.3389/fimmu.2021.752612. https://www.frontiersin.org/articles/10.3389/fimmu.2021.752612/full.