While it is possible to treat secondary breast cancer for some time, it currently cannot be cured. There are about 35,000 people currently living with secondary breast cancer, and 11,500 women die from the disease every year, with all of these due to breast cancer spreading.
When diagnosed early and confined to the breast tissue, it is a very successfully treatable disease, but unfortunately, many patients have a secondary disease when diagnosed, and others will develop metastasis during or after treatment. Even if not always possible to prevent, it is important to understand the factors that facilitate the process of metastasis so we understand how best to treat it.
Overall, research into why breast cancer spreads, how to stop it, and how to treat it effectively when it does is vital to ensure that we stop more women from dying.
Image Credit: Explode/Shutterstock.com
Why is the lung a common place for breast cancer to spread to? What effects does this cause on a person’s quality of life?
The lung is a common place for metastasis for many cancers. Even though breast cancer is frequently associated with bone metastasis, in some specific breast cancer subtypes (such as triple-negative, one of the most aggressive types), it is actually more frequently spreads to soft tissues such as the lungs, brain, and liver.
The lung is a very vascularized organ, meaning it has many densely packed small blood vessels, with the role of transporting oxygen to the body. Breast cancer cells that detach from the primary tumor often circulate in the bloodstream, and there is a high probability of getting trapped in these small lung capillaries.
Additionally, because lungs are in direct contact with the external environment (atmospheric air), they are naturally prone to inflammatory responses, which on one hand protect from external pathogens, but can also stimulate cancer growth. This means that cancer cells can rely on those conditions in the lung to grow and generate more tumors.
Lung tumors take up airspace, severely compromising the quality of life, with patients suffering from fatigue and breathlessness.
How are cancers able to spread to another part of the body?
To spread to another part of the body, cancer cells that detach from the primary tumor in the breast need to enter the bloodstream and, importantly, escape from the blood vessels at different organs, where they can enter the surrounding tissue and start growing in secondary tumors here.
However, this is a more challenging process for a cancer cell, and for that secondary tumor to form, the “healthy” organ must accept and allow the presence of the cancer cell, as well as its growth. So, the healthy cells within the secondary organ need to “help” the newly arrived tumor cell.
What is secondary breast cancer?
Can you describe how you carried out your research into the spread of breast cancer?
It has been known for a long time that HIF proteins (HIF-1a and HIF-2a) when expressed in tumor cells, make them more aggressive and more capable of spreading to other organs. These HIF proteins are important in normal cells, for survival and adaptation to low oxygen availability.
During my postdoctoral research, we saw that when one of these proteins (HIF-1a) was absent in the endothelial cells of mice (healthy cells that form small capillaries), they developed fewer lung tumors. Interestingly, the opposite was true when they were missing the other protein, HIF-2a, and developed many more.
Because these proteins develop important functions in normal healthy cells, we decided to investigate if the phenomenon was reversed when, instead of genetically deleting them, we activated them. So, we used hypoxia (low oxygen) to activate HIF-1a or HIF-2a and asked if having more or less of each of these proteins would affect the risk of developing lung tumors.
What did you discover?
We verified that too much HIF-1a in the endothelium made it more permeable or porous, which made it easier for cancer cells to exit the blood circulation into the lung tissue. Also, having more HIF-1a created an inflammatory environment that favored the growth of these cells in the lung tissue, meaning they more easily grew into tumors.
When there was more HIF-2a, this was not the case. That is because HIF-2a is involved in normal endothelial cell function and its stability as a barrier for the cancer cells inside the vessels, so having more HIF-2a in the endothelium makes it less likely that cancer cells infiltrate the lungs and form tumors.
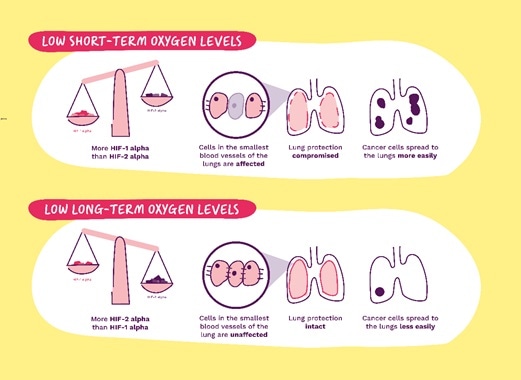
Image Credit: https://breastcancernow.org/
What is the everyday role of the two proteins you discovered (HIF-2α and HIF-1α)?
As widely disseminated following the Nobel Prize in Medicine and Physiology in 2019, these proteins are like molecular switches in all cells, that perceive oxygen levels and help them adjust to the changing oxygen levels and survive. They are important in metabolism and cellular energy, but over the years other functions have been associated with them.
HIF-1a is primarily associated with stress responses and is activated quickly and transiently (on and shortly after, off), whereas HIF-2a has a slower and more persistent activation (takes longer to be turned on and remains active for longer). Specifically, in endothelial cells, HIF-2a is usually present to maintain the stability of capillary networks, so removing it from these cells, in particular, is not beneficial in the context of cancer metastasis.
How does the HIF-1α protein make it easier for cancer cells to move?
When expressed in cancer cells, as mentioned above, the activation of this protein results in cells being more resistant and more mobile, with more cells infiltrating the surrounding tissue and likely to migrate to organs away from the primary tumor. When expressed in endothelial cells, it can cause them to proliferate more (make more blood vessels), become more permeable (more gaps between the cells), so it becomes easier for cells in the bloodstream to move in and out.
Additionally, endothelial cells with activated HIF-1a generate pro-inflammatory signals or compounds, which means inflammatory cells are also activated; This is important and useful in normal conditions, such as during pathogen invasion, exercise, or high altitude, but in the context of cancer, increased permeability and inflammation are “pro-metastatic”, meaning these responses actually favor the formation of secondary tumors.
Why was it useful to be able to study how non-cancer cells behave during the spread of cancer?
Traditional therapies are aimed at eliminating cancer cells. Unfortunately, some cancer cells escape those treatments and remain in the body.
We know that the only way those resistant cancer cells can survive and grow into new tumors is with the cooperation of the non-cancer cells; if we understand how those normal cells behave in helping cancer, treatments can be developed to target those interactions and prevent secondary cancer by making the normal tissues “unhelpful” to the secondary cancer growth.
Do you believe that your research will help us to further understand the effects of breast cancer?
I believe secondary breast cancer needs to be at the top of the priorities in breast cancer research in general. Great investments have been made in early diagnosis and treatment of primary tumors, and to great benefit to the patient community.
However, the secondary breast cancer patient groups do not have effective treatments available to them, and alternative or additional strategies must be developed to transform the outcome of patients living with secondary breast cancer.
The research in my lab is effectively addressing this, and we aim to contribute to a critical void in identifying patients at risk for metastasis, as well as treatment options for patients living with metastasis.
Why can we currently not cure secondary breast cancer? How could your research be utilized to help develop an effective treatment/therapy for secondary breast cancer?
Metastatic cancer is made of very resilient cells, which are hard to eliminate: these are cells that acquired the ability to migrate, and often are also the ones that escaped the initial treatments, so they tend to be resistant to standard treatments and chemotherapy. This means that even though the normal treatments can sometimes delay cancer spread, they cannot reverse metastasis, so it is, as of now, an incurable condition.
The therapeutic strategies available continue to rely on well-known and powerful drugs that aim to kill the tumor cells, but currently, there are no drugs or treatments that prevent or stop the support that the healthy cells provide to the secondary cancers.
Research into the behavior of those healthy cells in the process of forming secondary tumors will help understand how those behaviors can be stopped or interrupted, and thus the support for those secondary tumors removed.
Image Credit: Lightspring/Shutterstock.com
How could your research help to identify the patients most at risk from developing secondary breast cancer?
This is a small contribution to a very big problem.
Identifying the conditions that make secondary cancer more likely can be used as parameters to screen and look for when patients come into the clinic.
What are the next steps in your research into breast cancer?
Most of these studies have been done looking into secondary cancer to the lung, and we are interested in investigating if the processes we identify here are the same in other tissues where breast cancer patients develop secondary tumors, to see if those are widespread or specific to the lung.
We will do this by also looking into tissues where breast cancer does not cause secondary tumors, to understand what could offer that advantage or resistance, and possibly further exploit that therapeutically.
In other words, we want to know what makes some organs vulnerable, and others strong against developing secondary tumors. We are also interested in understanding the impact of cancer treatments in non-cancer cells, and how those may affect long term survivorship.
Where can readers find more information?
Institutional Webpage: https://pure.qub.ac.uk/en/persons/cristina-branco
Laboratory webpage: https://cristinabrancoqub.wixsite.com/brancogroup
About Dr. Cristina Branco
Dr. Cristina Branco received her Ph.D. in Biology in 2008 from the New University of Lisbon, following a research program at the University of California, Riverside. She did her postdoctoral training in the Department of Biological Sciences at UC San Diego and the Department of Physiology, Development, and Neuroscience at the University of Cambridge, and studied the contribution of endothelial cells to metastasis in Breast Cancer while collaborating on other projects linked to oxygen responses and vascular function.
In 2014, Dr. Branco received a Scientific Fellowship from Breast Cancer Now to further explore the role of HIF transcription factors and hypoxia in breast cancer metastasis. She was appointed Lecturer in Metastasis at Queen’s University Belfast in 2018, where she currently runs a research lab studying organ-specific vascular and perivascular responses to cancer progression in pre- and early metastasis, primarily in models of triple-negative breast cancer.