As the coronavirus disease (COVID-19) pandemic continues to rage across the globe, many countries have rolled out vaccines. Populations to be vaccinated first are healthcare workers, front-liners, and high-risk individuals. The majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines in use or advanced clinical development are based on the viral spike protein (S) as their immunogen.
Since the start of the pandemic, there are now 235 vaccine candidates for SARS-CoV-2. Of these, 172 vaccines are in pre-clinical evaluation, while 63 are undergoing human trials. Sixteen vaccines are in the last phase of human trials, with a handful receiving emergency use authorization.
Now, researchers at the Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom, reveal that engineered closed S constructs may be valuable immunogens that can induce neutralizing antibodies against a different pattern of epitopes. This allows for the flexibility of vaccines at both the individual and population levels.
The S protein is present in viruses wherein the receptor-binding domain (RBD) is open or closed. However, it has been described that neutralizing antibodies act against both open and closed RBDs. The long-term efficacy and safety of vaccines rely on inducing these antibodies to protect the evolving and circulating SARS-CoV-2 strains.
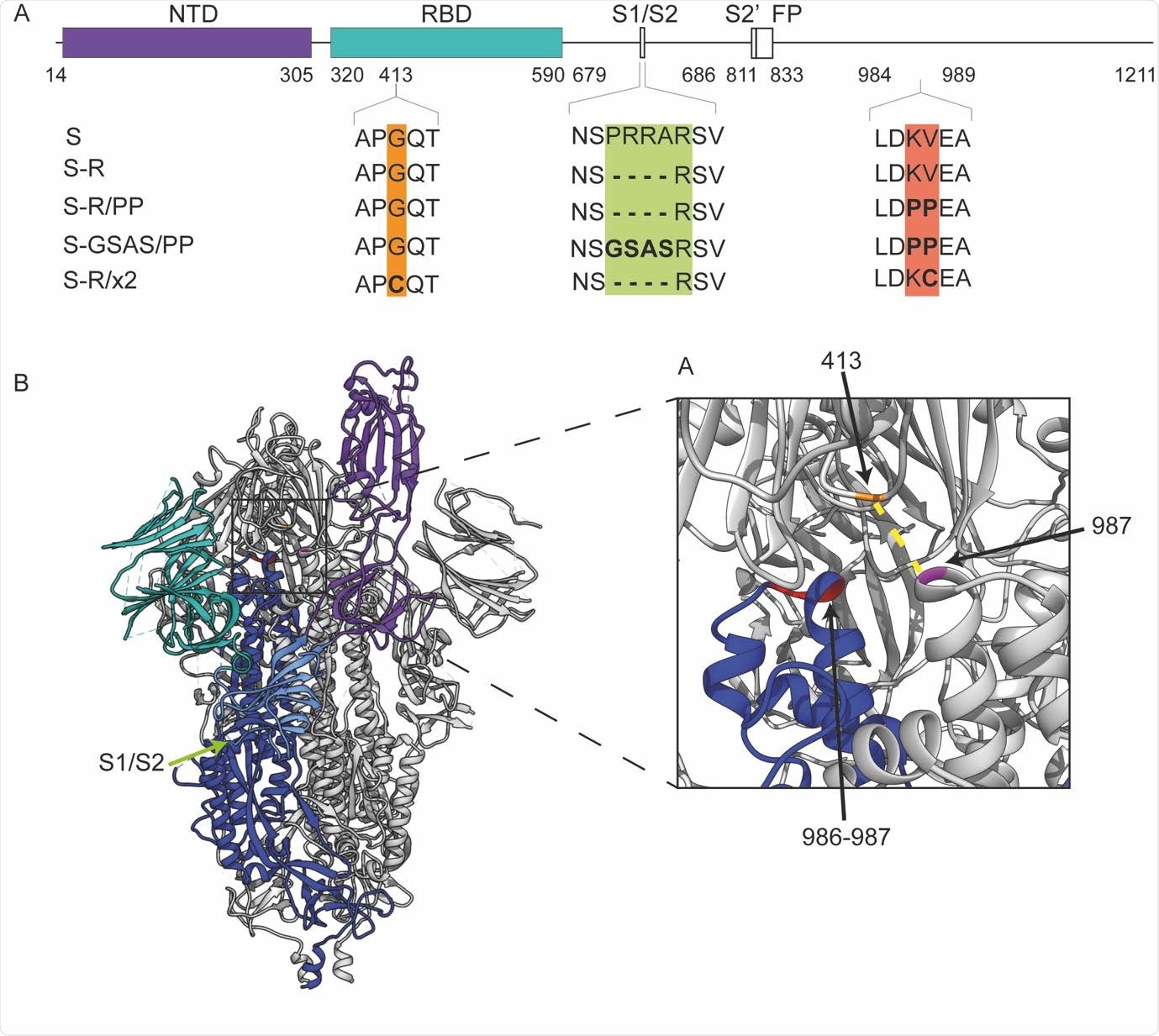
Design of constructs. A. Overview of constructs used in the study indicating the positions where residues have been mutated. B. An overview of the trimeric spike structure indicating the positions of the mutated residues. The insertion of cysteine residues at positions 413 and 987 leads to the formation of a disulphide bond (dotted yellow line in inset) that arrest S in the closed prefusion form.
The study
In the study, posted on the pre-print server bioRxiv*, the team assessed the results of immunization in mouse models using an S protein trimer in the closed state to prevent exposure of the RBD site.
To arrive at the study findings, the researchers developed S protein constructs that are in the closed perfusion conformations, where the RBD should not be accessible to the angiotensin-converting enzyme 2 (ACE2) receptor site, the cellular gateway of SARS-CoV-2.
The team theorized that closed spikes would provide a different antibody response compared to standard S protein constructs. The team immunized ice with various S protein constructs that differ in containing the double proline mutant, the mutation existing at the furin cleavage site.
The study findings suggest that S trimers arrested in the close state can be used to develop future and next-generation SARS-CoV-2 vaccines. These vaccines can help trigger broader and more robust neutralizing antibody responses.
Further, the team also found that when they obtained serum samples from the mice, most mice vaccinated with S variants had neutralizing sera two weeks after the immunization. At two weeks after the vaccination, the serum samples were potently neutralizing. The serum samples also remained potently neutralizing 21 weeks after vaccination.
Immunization of mice with trimeric S protein constructs induces very strongly neutralizing sera, and the sera remain neutralizing 5 months post-immunization with neutralizing antibody titers maintained at high levels. Immunization also induces a T-cell response. All of the sera contain antibodies that bind S and RBD, and which block the ACE2- RBD interaction, including sera induced by the stabilized, closed S-R/x2 construct.,” the team summarized in the paper.
Currently, the S proteins expressed by vaccines such as the Pfizer/BioNTech and Moderna messenger ribonucleic acid (mRNA) vaccines, the AstraZeneca ChAd vaccine, and others, are all based on early S proteins designs that can adapt to both and closed conformations.
The COVID-19 toll
To date, the global case toll of COVID-19 has reached 95.48 million, and the virus has now reached 191 countries and regions. The death toll has surpassed 2 million, while the United States remains the nation with the highest number of cases, topping 24 million. The country has recorded over 398,000 deaths since the pandemic arrived in the country in January.
The other countries that have reported skyrocketing COVID-19 cases include India, with over 10.57 million cases, Brazil, with more than 8.48 million cases, Russia, with over 3.55 million cases, and the United Kingdom, with more than 3.44 million cases, among others.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Source:
Journal references:
- Preliminary scientific report.
Carnell, G., Ciazynska, K., Wells, D., et al. (2021). SARS-CoV-2 spike protein arrested in the closed state induces potent neutralizing responses. bioRxiv. https://www.biorxiv.org/content/10.1101/2021.01.14.426695v1
- Peer reviewed and published scientific report.
Carnell, George W., Katarzyna A. Ciazynska, David A. Wells, Xiaoli Xiong, Ernest T. Aguinam, Stephen H. McLaughlin, Donna Mallery, et al. 2021. “SARS-CoV-2 Spike Protein Stabilized in the Closed State Induces Potent Neutralizing Responses.” Edited by Colin R. Parrish. Journal of Virology 95 (15). https://doi.org/10.1128/jvi.00203-21. https://journals.asm.org/doi/10.1128/JVI.00203-21.