The inhibition of pathological protein-protein interactions is a promising approach for treating a large number of diseases, including many forms of cancer. A team of researchers has now developed a bicyclic peptide that binds to beta-catenin--a protein associated with certain types of tumor. The secret of their success is the cyclic nature and the hairpin shape of the peptide, which mimics a natural protein structure, they report in the journal Angewandte Chemie.
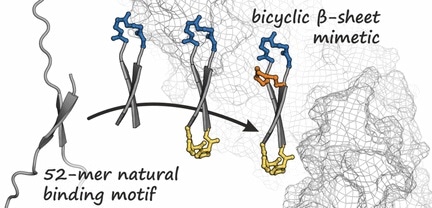
Image Credit: 'Angewandte Chemie'
Because of the extensive protein regions involved in protein-protein interactions, therapeutic approaches involving small molecules are often unsuccessful. Protein mimetics are alternatives that imitate the spatial structure of binding segments of natural protein binding partners. Although beta-sheets--protein structures made of several stretched out peptide chains arranged side by side, resembling a sheet of paper folded like an accordion--often play a role in the interaction of proteins, they have rarely been used as a basis for mimetics. This is partly because they have problems entering the target cell, and thus, cannot reach the pathogenic protein.
Led by Tom N. Grossmann, an international team from the Vrije Universiteit Amsterdam (Netherlands), Università degli Studi di Napoli "Federico II" (Italy), as well as AstraZeneca (Cambridge, UK), has now reported the design of β-sheet mimetics that inhibit the intracellular oncogenic protein beta-catenin. beta-Catenin is a component of the Wnt signaling pathway and activates T-cell factors (TCF), which ultimately stimulate cell growth and proliferation. Hyperactivation of the Wnt pathway is associated with various forms of cancer. Inhibition of the interaction between beta-catenin and TCF is thus an appealing therapeutic approach.
Based on the known structure of beta-catenin when it is in a complex with a protein, the team first produced a binding partner for beta-catenin. This partner is a ring-shaped peptide that forms a short, antiparallel beta-sheet--known as a beta-hairpin structure--when it is bound to beta-catenin, as demonstrated by an analysis of its crystal structure.
The idea was to fix this cyclic peptide in the hairpin form by introducing an additional bridge. This generates a bicyclic structure that strengthens binding to beta-catenin. By using a series of different synthesized variants, the team was able to identify several bicyclic peptides with a high affinity for beta-catenin. Among these, they found a compound that (other than the original cyclic peptide) successfully penetrates cells and significantly inhibits the oncogenic Wnt signal cascade.
This newly developed bicyclic beta-sheet mimetic thus represents a possible starting point for the development of new antitumor drugs that inhibit cellular Wnt signaling. This strategy could also be used for the design of further inhibitors of other protein-protein interactions mediated by beta-sheets.
Source:
Journal reference:
Wendt, M., et al. (2021) Bicyclic β‐Sheet Mimetics that Target the Transcriptional Coactivator β‐Catenin and Inhibit Wnt Signaling. Angewandte Chemie International Edition. doi.org/10.1002/anie.202102082.