COVID-19 has imposed a massive global health and economic crisis of unprecedented proportions. Despite ongoing mass vaccination programs, the pandemic is still causing considerable morbidity and mortality, which highlights the need for more effective therapies.
Human monoclonal antibodies (mAbs) that can specifically target viral surface proteins have been shown to demonstrate prophylactic and therapeutic efficacy against many viruses, including Ebola, HIV, beta-coronaviruses MERS-CoV, SARS-CoV-1, and now SARS-CoV-2.
Using passively administered neutralizing antibodies is a promising approach for the prevention and treatment of SARS-CoV-2 infection. Many neutralizing antibodies were isolated over the last year, all of which targeting the SARS-CoV-2 spike protein, its receptor-binding domain, and N-terminal domain.
Despite this, the role of Fc-mediated functions in the effective in vivo neutralization of SARS-CoV-2 is not well understood. Only a limited number of studies have focused on this area so far. Recent studies reported that by improving the functionality of the Fc, the in vivo activity of a partially neutralizing antibody can be considerably improved.
Since anti-SARS-CoV-2 antibody-based therapy helps treat COVID-19 patients or is being used prophylactically to immunocompromised populations, it is imperative that further investigations are done on the role of Fc role in the neutralization of SARS-CoV-2. The role played by Fc activation will be a significant factor in the design of therapeutic antibodies.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Comparing protective activity of Fc-active and Fc-inactive neutralizing antibodies against lethal SARS-CoV-2 infections in transgenic mice
Researchers from Israel recently isolated a set of SARS-CoV-2 neutralizing monoclonal antibodies from blood samples of patients with severe COVID-19. They were the first to show that transgenic K18-hACE2 mice infected with lethal doses of SARS-CoV-2 can be fully protected by administering antibodies even 3 days after infection. This demonstrates the excellent potency of these antibodies. In this study, they hypothesized that Fc-effector functions play only a diminished role in the protective activity of potent SARS-CoV-2 neutralizing antibodies. This study is published on the preprint server, bioRxiv.*
The researchers engineered the Fc of two potent SARS-CoV-2 neutralizing human monoclonal antibodies that target distinct domains of the spike protein to rescind their Fc-dependent functions. The protective activity of these neutralizing antibodies was assessed against lethal SARS-CoV-2 infections in K18- hACE2 transgenic mice, before or two days post-exposure and compared to their original, Fc-active antibodies.
Results show that “elite” antibodies do not require immune system modulation
The results showed that the protective activity of antibody treatment with both Fc variants was similar. Both Fc variants reduced viral load, decreased morbidity, and rescued the mice from death. Additionally, surviving animals developed a potent endogenous immune response to the virus. Thus, the findings show that the two highly potent SARS-CoV-2 neutralizing antibodies do not depend on Fc-mediated immune activation to fully protect infected K18- hACE2 mice against lethal SARS-CoV-2 infections.
“Antibody treatment with both Fc-variants similarly rescued the mice from death, reduced viral load and prevented signs of morbidity.”
In summary, many studies have shown that the in vivo potency of anti-SARS-CoV-2 antibodies relies on Fc-effector functions in the post-exposure treatment of SARS-CoV-2. They also showed that the activation of these pathways could improve the neutralizing potency of these antibodies. For the first time, the researchers demonstrate a proof-of-concept that “elite” antibodies do not require immune system modulation.
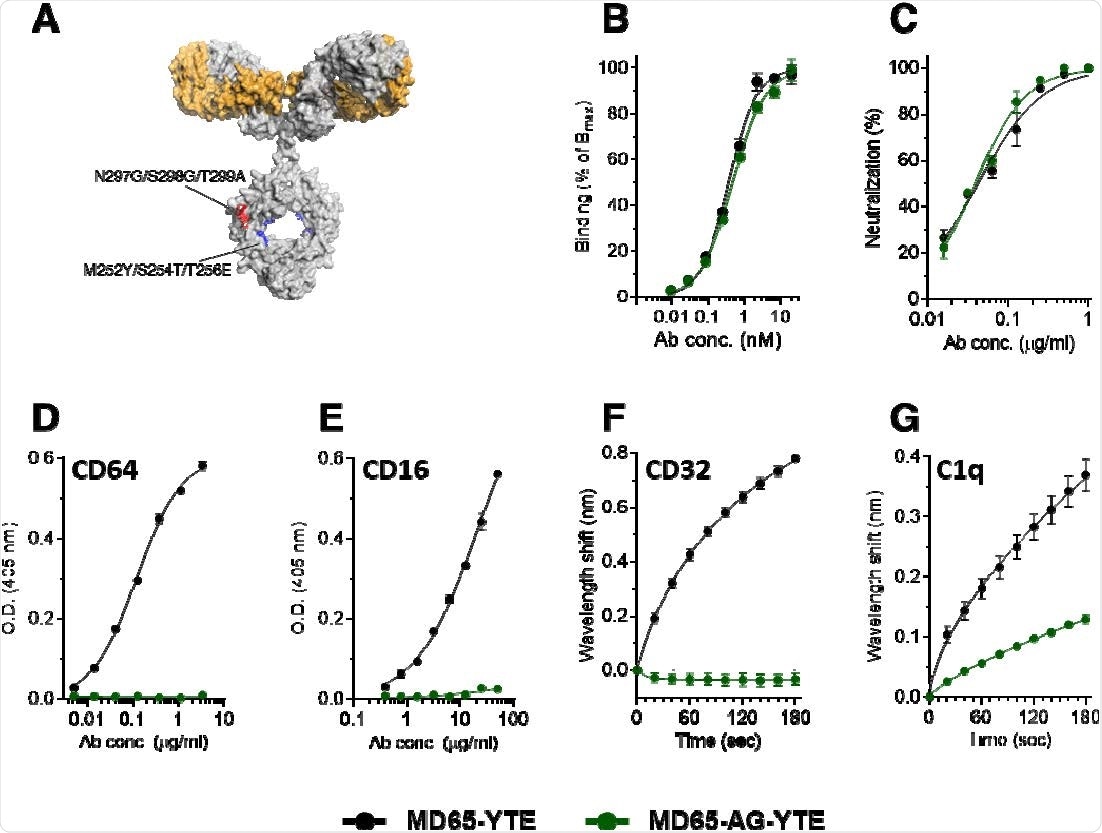
Binding characteristics of the MD65 antibody engineered versions. (A) Schematic representation of the mutations inserted into the YTE version (Red) and the AG-YTE version (Red and Blue). (B) Binding to immobilized SARS-CoV-2 spike protein, tested by ELISA. (C) In vitro neutralization of SARS-CoV-2 using plaque reduction neutralization test (PRNT). (D, E) Binding to immobilized CD64 (D) or CD16 (E), evaluated by ELISA. (F) BLI measurements of the binding of each antibody to immobilized CD32. (G) BLI measurements of the binding of C1q to the immobilized antibodies. Values are average ± SEM of triplicates of representative experiment (B-E) or of three independent experiments (F, G).
As the activation of the immune system can be beneficial even for these antibodies and since there is no clear antibody-dependent enhancement of disease in the treatment of COVID-19, the ability to activate Fc-effector functions should be maintained. Despite this, as anti-SARS-CoV-2 antibody-based therapy is designed to treat immunocompromised COVID-19 patients, the role of Fc-activation should be analyzed for each antibody before it is used in the clinic.
“Here, we demonstrate for the first time, a proof-of-concept that “elite” antibodies do not necessarily require the modulation of the immune system.”
Overall, this study provides critical insights into the contribution of Fc-effector functions in antibody-mediated protection against SARS-CoV-2, which could help the design of effective antibody-based therapies for COVID-19 in the future.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Fc-independent neutralization of SARS-CoV-2 by recombinant human monoclonal antibodies, Tal Noy-Porat, Avishay Edri, Ron Alcalay, Efi Makdasi, David Gur, Moshe Aftalion, Yentl Evgy, Adi Beth-Din, Yinon Levy, Eyal Epstein, Olga Radinsky, Ayelet Zauberman, Shirley Lazar, Shmuel Yitzhaki, Hadar Marcus, Angel Porgador, Ronit Rosenfeld, Ohad Mazor, bioRxiv, 2021.05.15.443978; doi: https://doi.org/10.1101/2021.05.15.443978, https://www.biorxiv.org/content/10.1101/2021.05.15.443978v1
- Peer reviewed and published scientific report.
Noy-Porat, Tal, Avishay Edri, Ron Alcalay, Efi Makdasi, David Gur, Moshe Aftalion, Yentl Evgy, et al. 2021. “Fc-Independent Protection from SARS-CoV-2 Infection by Recombinant Human Monoclonal Antibodies.” Antibodies 10 (4): 45. https://doi.org/10.3390/antib10040045. https://www.mdpi.com/2073-4468/10/4/45.