A team of scientists from Germany has recently evaluated the reactogenicity profile of heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination among healthcare workers. The findings reveal that a 12-week interval regimen of heterologous vaccination is well-tolerated among participants. The study is currently available on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is by far the largest pandemic in modern history, with over 167 million confirmed infections and more than 3.4 million deaths worldwide. The only possible way to control the pandemic is rapid and widespread vaccination. To meet the increasing demand for vaccines or to overcome vaccine shortages, heterologous prime-boost vaccination is becoming the topic of international interest.
Because of rare blood clotting events associated with the Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19), healthcare advisory bodies of many countries are now recommending that people previously vaccinated with ChAdOx1 nCoV-19 should receive a second booster dose of most preferably mRNA-based vaccines, such as BNT162b2 (Pfizer-BioNTech). However, not enough data is currently available on the immunogenicity, reactogenicity, and safety of heterologous prime-boost vaccination regimen.
In the current study, the scientists have investigated the reactogenicity of homologous BNT162b2/BNT162b2 and heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination regimens in a group of 326 healthcare workers from Berlin, Germany.
Study design
In the entire study cohort, the reactogenicity data after the first vaccination with BNT162b2 or ChAdOx1 nCoV-19 were available for 178 and 148 participants, respectively. Similarly, the reactogenicity data after homologous BNT162b2/BNT162b2 and heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccinations were available for 159 and 71 participants, respectively.
In the heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination group, two doses of respective vaccines were administered at an interval of 12 weeks.
The US Food and Drug Administration (FDA) modified criteria were used to grade vaccine-associated reactogenicity.
Important observations
No significant difference in frequency or severity of local reactions was observed after homologous or heterologous prime-boost vaccination. However, considerable differences in systemic reactions were observed between the tested vaccination regimens. The most frequent systemic reactions were observed after prime vaccination with ChAdOx1 nCoV-19 (86%), followed by homologous BNT162b2/BNT162b2 (65%) and heterologous ChAdOx1 nCoV-19/BNT162b2 (48%) prime-boost vaccinations.
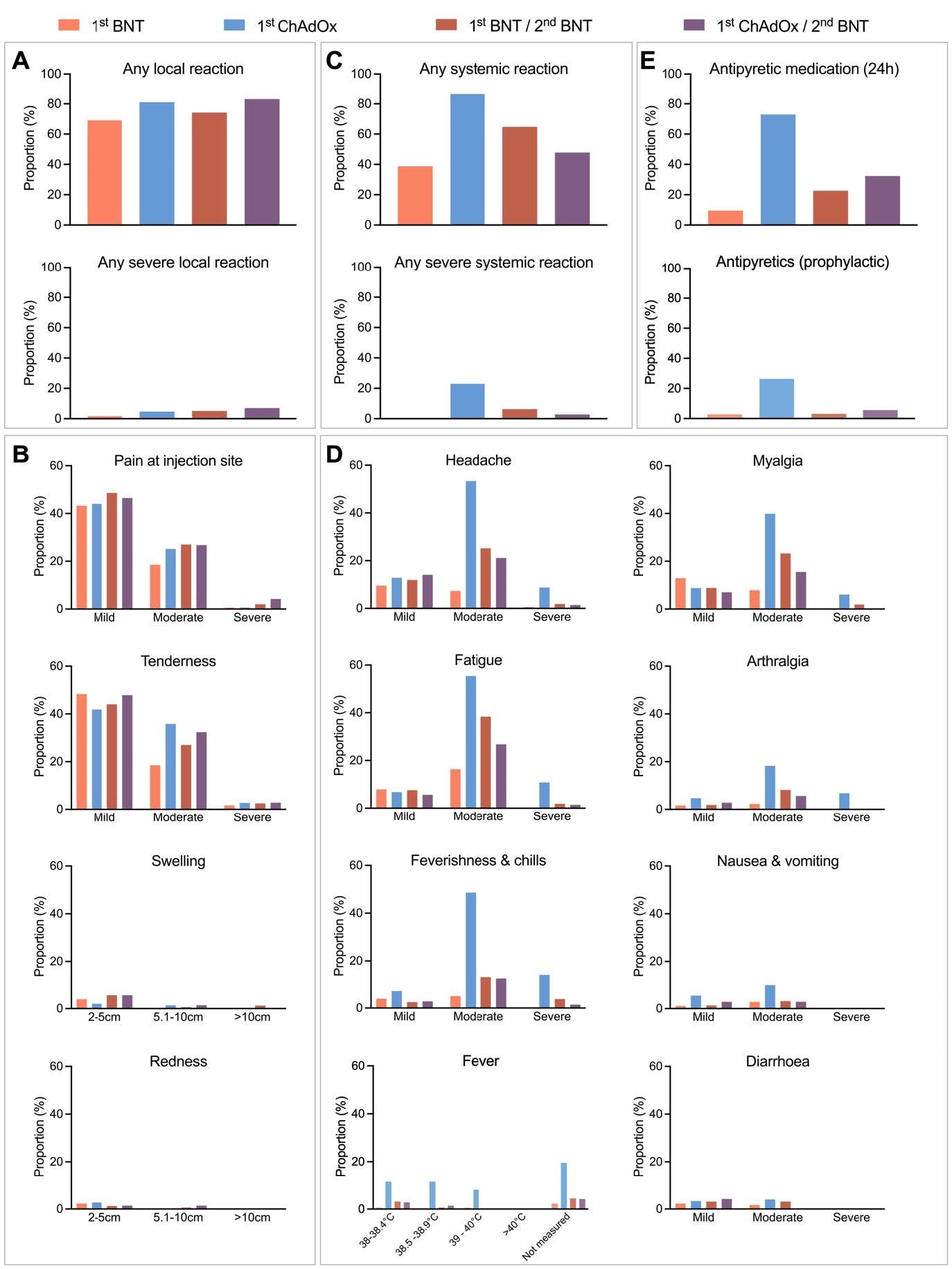
Solicited local and systemic reactions of different vaccine regimens reported until seven days after vaccination. (A, B) Proportion of participants reporting any local reaction (A), and indicated local reactions grouped by severity (B). (C, D) Proportion of participants reporting any systemic reaction (C), and indicated systemic symptoms grouped by severity (D). (E) Proportion of participants reporting intake of antipyretic medication within 24 hours (top) and seven days (bottom) after vaccination. BNT; BNT162b2 (Comirnaty), ChAdOx; ChAdOx1- nCoV19 (Vaxzevria). Definition of severity according to a modified Food and Drug administration (FDA) criteria.
Compared to heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination, ChAdOx1 nCoV-19 prime vaccination was associated with more frequent severe systemic reactions including fatigue, myalgia, headache, chills, and high fever. The highest intake of antipyretic medicines was observed before (protective) and after (curative) ChAdOx1 nCoV-19 prime vaccination. In contrast, significantly lower consumption of antipyretic medicines was observed among participants belonging to other vaccination groups.
Study significance
The study findings reveal comparable reactogenicity for homologous BNT162b2/BNT162b2 and heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccinations. Among study participants, heterologous prime-boost vaccination exhibits a well-tolerated profile.
Based on the study findings, the scientists recommend that immunizing people with the 1st dose of ChAdOx1 nCoV-19 vaccine and 2nd dose of BNT162b2 vaccine at an interval of 12 weeks can significantly reduce vaccine-associated reactogenicity.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sander L et al., 2021. Reactogenicity of homologous and heterologous prime-boost immunization with BNT162b2 and ChAdOx1-nCoV19: a prospective cohort study. medRxiv. https://www.medrxiv.org/content/10.1101/2021.05.19.21257334v1
- Peer reviewed and published scientific report.
Hillus, David, Tatjana Schwarz, Pinkus Tober-Lau, Kanika Vanshylla, Hana Hastor, Charlotte Thibeault, Stefanie Jentzsch, et al. 2021. “Safety, Reactogenicity, and Immunogenicity of Homologous and Heterologous Prime-Boost Immunisation with ChAdOx1 NCoV-19 and BNT162b2: A Prospective Cohort Study.” The Lancet Respiratory Medicine 0 (0). https://doi.org/10.1016/S2213-2600(21)00357-X. https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(21)00357-X/fulltext.