Porvair Sciences reports investment in a dedicated clean area for manufacturing Vyon® porous plastic components for healthcare, pharmaceutical and life science products.
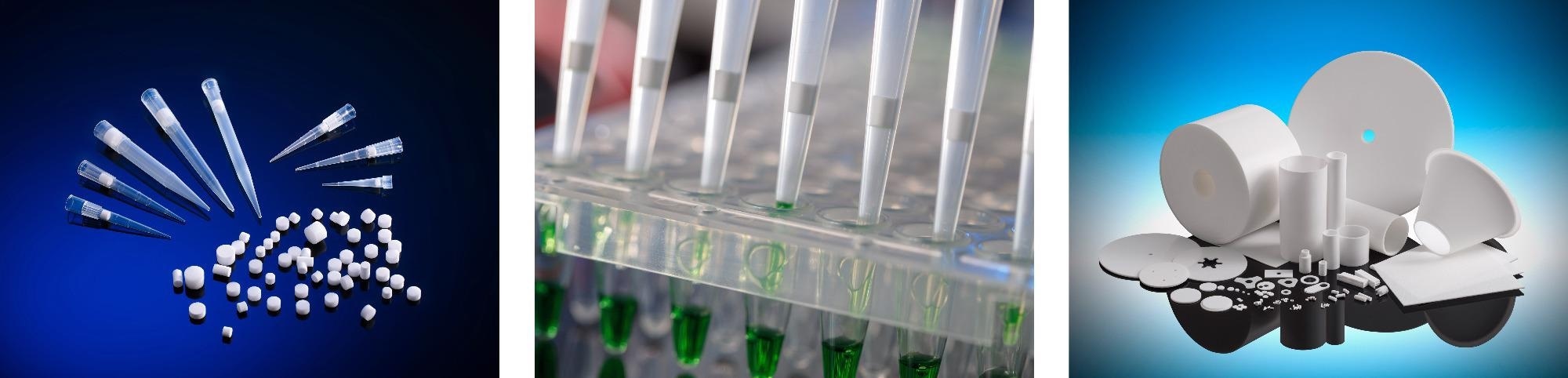
Image credit: Porvair Sciences
With the global market searching for high performance solutions for critical filtration, laboratory liquid handling and separation applications - Porvair has experienced a doubling in demand for components and products such as pipette filter tips based upon its Vyon® porous plastics.
Kevin Doolan, General Manager of Porvair Sciences said "Our new Vyon white room was built in response to increasing customer requests for a controlled manufacturing environment, dedicated to fulfilling the strict regulatory & cleanliness requirements for components used in their products. Within the white room we have implemented cGMP compliant procedures enabling us to achieve even tighter specifications for contamination-free components ".
Drawing upon decades of knowledge in the manufacture of industry-leading porous plastics, combined with advanced materials expertise, Porvair Sciences has established a reputation for delivering outstanding components to OEM & laboratory customers worldwide. Proprietary to Porvair Sciences - Vyon® is a highly versatile porous plastic which can be modified to give hydrophilic or hydrophobic properties, or enhanced to achieve specific chemical and biochemical separations. Vyon® can also be combined with other materials to create application specific composite products. These and other desirable properties have made Vyon® the porous plastic material of choice for healthcare, pharmaceutical and life science companies looking to produce filters, vents, bed supports and sample separation products with a competitive advantage. Vyon® is available in a range of shapes and sizes to suit your needs.
To discuss how our Vyon® porous plastic materials can help improve your products performance, please contact Porvair Sciences Ltd on +44-1978-661144, [email protected] or visit https://www.vyonporousplastics.com/ for more information.