Recent observations show that the effectiveness of COVID-19 vaccines has decreased because of the emergence of the Delta variant and the waning of vaccine-induced immunity. This has led to a campaign in many countries to provide booster doses of COVID-19 vaccines.
A register-based cohort study of the effectiveness of COVID-19 vaccines was performed to help decision-making on providing booster doses to social and healthcare workers (HCWs) like nurses, physicians, dentists, and other professionals in Finland. In addition, it estimated the vaccine effectiveness after the second dose. This study is published on the medRxiv* preprint server.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Overview of the study
This study was carried out with the aim to assess whether the shield against COVID-19 weakens in HCWs after both the doses of either messenger RNA (mRNA) or adenovirus vector (AdV) vaccines and after a first AdV and second mRNA vaccine during the first ten months of the vaccination drive in Finland.
HCWs within the age range of 16-69 years were vaccinated with mRNA vaccines – Comirnaty (BioNTech, Pfizer) and Spikevax (Moderna) – at the standard 3-4 week dosing interval. However, in the case of the AdV vaccine, Vaxzevria (Oxford, AstraZeneca), the dosing interval was prolonged to 12 weeks.
This study cohort comprised 427,905 HCWs including 291,758 or 68% nurses, 23,886 or 6% physicians, 13,074 or 3% dentists and oral hygienists, and 99,187 or 23% other health care professionals.
At least 90% of the HCWs received at least one dose of the vaccine by the end of follow-up. Two doses of mRNA vaccines and AdV vaccines were received by 315,413 (74%) and 14,760 (3%) HCWs, respectively. In addition, the heterologous vaccines were given to 30,548 (7%) HCWs.
Study results
The study results showed that 3,874 HCWs got infected with SARS-CoV-2 in the unvaccinated group, while 1,757 HCWs were infected in the vaccinated group.
On further analysis, it was noted that after 14-90 days of the second dose, vaccine effectiveness against infection was 82% for mRNA vaccines, 89% for AdV vaccines, and 80% for the combination vaccine series.
However, after 91-180 days from the second dose, effectiveness decreased to 62% for mRNA vaccines, 63% for AdV vaccines, and 62% for the combination vaccine series.
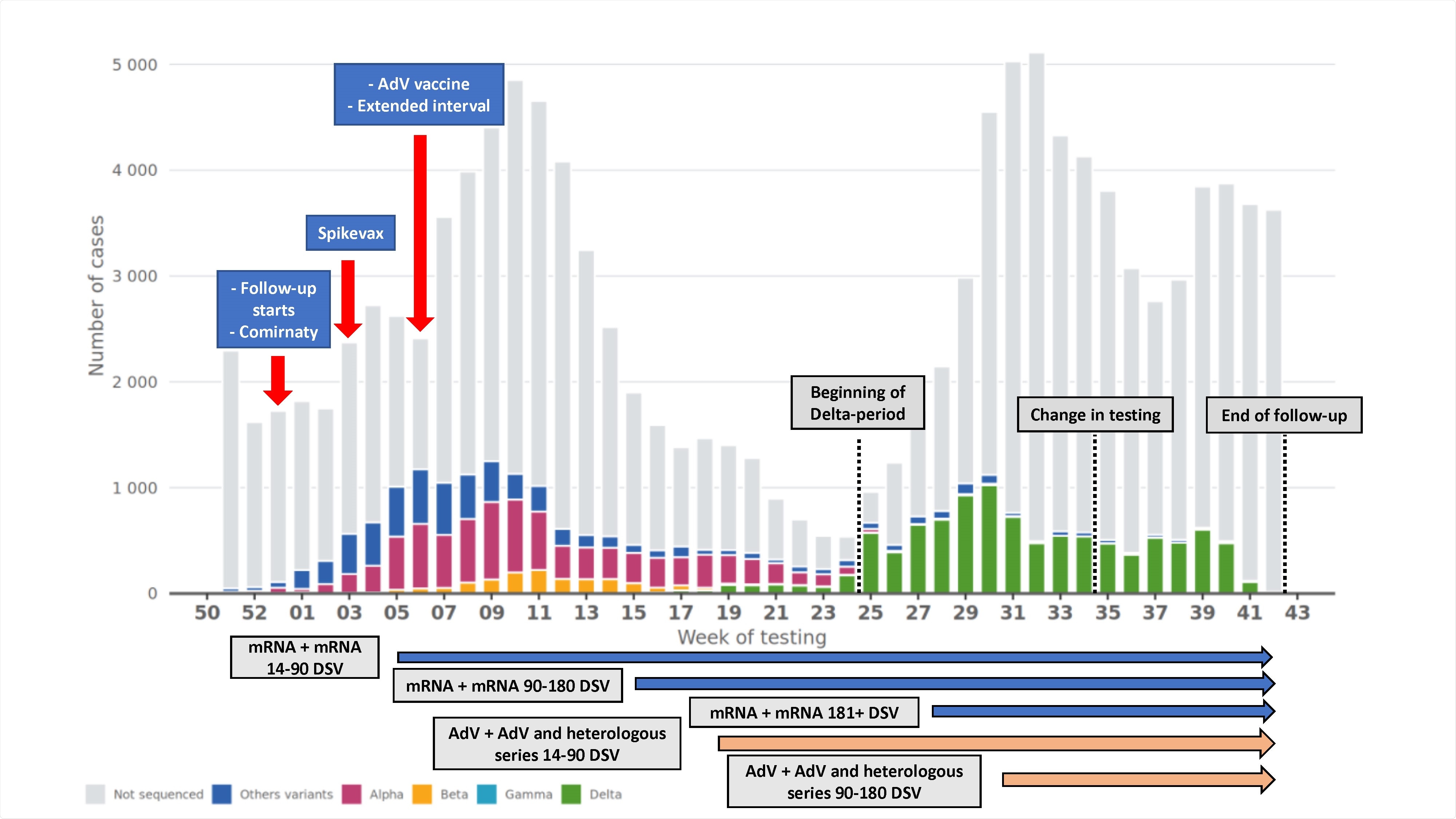
Furthermore, in the unvaccinated group, there were 220 cases of COVID-19-related hospitalization compared to 35 cases in the vaccinated group. During the first ten months of the vaccination drive, it was noted that all vaccine series were 88% or more effective against hospitalization.
“Boosters may be beneficial for healthcare workers to enhance protection against infection and to decrease transmission of SARS-CoV-2 to patients.”
Main findings
The authors observed that the protection against SARS-CoV-2 infection was high at the early stages after the second dose; however, the efficacy decreased after three months.
Further, the protection against COVID-19 hospitalization was strong beyond six months. Also, the protection provided by both doses of either mRNA or AdV vaccines and that after a heterologous AdV + mRNA vaccine was similar.
Additionally, no change in vaccine effectiveness was observed due to the appearance of the Delta variant which indicates that the decrease in vaccine effectiveness is due to diminishing vaccine-induced immunity.
Limitations of the study
The few limitations of this study include the inability to thoroughly evaluate the effectiveness of vaccines in HCWs working in intensive care units six months after the second dose.
Also, in one group, the second dose was given 3-4 weeks after the first one, and in another group, it was administered 12 weeks after the first one. This difference in dosing interval leads to a biased estimation of vaccine effectiveness as extended dosing interval elicits a more significant antibody response.
Furthermore, the vulnerability of the work area of HCWs was not considered in this study, which precludes the assessment of work-related infection.
Conclusion
Based on the findings of this register-based cohort study, the authors suggest that boosters may be helpful for HCWs to increase protection against SARS-CoV-2 infection that would also help reduce the transmission of the virus to patients.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020 - October 2021 Eero Poukka, Ulrike Baum, Arto A Palmu, Toni O Lehtonen, Heini Salo, Hanna Nohynek, Tuija Leino medRxiv 2021.11.03.21265791; doi: https://doi.org/10.1101/2021.11.03.21265791, https://www.medrxiv.org/content/10.1101/2021.11.03.21265791v2
- Peer reviewed and published scientific report.
Poukka, Eero, Ulrike Baum, Arto A. Palmu, Toni O. Lehtonen, Heini Salo, Hanna Nohynek, and Tuija Leino. 2021. “Cohort Study of Covid-19 Vaccine Effectiveness among Healthcare Workers in Finland, December 2020 - October 2021.” Vaccine, December. https://doi.org/10.1016/j.vaccine.2021.12.032. https://www.sciencedirect.com/science/article/pii/S0264410X21016406.