While the regenerative capacity after any injury differs across species, wound healing invariably results in a scar. Skin fibrosis or scar formation is a multifactorial process involving specific dermal fibroblast activation and inflammation. Both angiogenesis during wound healing and increased capillary levels at the wound site are also associated with increased scar formation.
Fibroblasts are crucial during wound healing and remodeling processes, during which these cells deposit the extracellular matrix at the wound site. Likewise, MFAP5, w fibroblast-derived mediator, plays a significant role in dermal healing.
MFAP5 influences microfibril function by interacting with fibrillin and regulating Notch signaling and the bioavailability of transforming growth factor beta (TGF-β). However, while the extracellular matrix and its role in scar formation have been studied extensively, the role of microfibril-associated proteins, which are abundant in the extracellular matrix of mature and developing tissues, in wound healing remains understudied.
About the study
In the present study, researchers isolated phagocytic fibroblasts by incubating neonatal human dermal fibroblasts with apoptotic microvascular endothelial cells from the human dermis, as previous studies have shown that fibroblasts exhibit a pro-fibrotic phenotype after ingesting apoptotic cells through phagocytosis. Ribonucleic acid (RNA) sequencing was conducted to examine the proteins expressed by the phagocytic fibroblasts.
Apoptotic endothelial cells were tagged with a green fluorescent protein (GFP). Subsequently, fluorescence-activated cell sorting (FACS) was used to separate phagocytic fibroblasts from non-phagocytic fibroblasts. Differential expression and RNA sequencing analyses were used to compare the transmembrane proteins produced by phagocytic and non-phagocytic fibroblasts.
The researchers used mouse models to study the expression of MFAP5 during the remodeling and proliferation phases of wound healing by determining the expression of the Mfap5 gene in the wound tissue using reverse transcription polymerase chain reaction (RT-PCR). Cultured phagocytic fibroblasts were also analyzed through RT-PCR.
Mice were randomly assigned to groups comprising treatment with the negative control phosphate-buffered saline (PBS), unconjugated mouse immunoglobulin G (IgG) isotype control antibody, or a monoclonal antibody against MFAP5 to determine the role of MFAP5 in angiogenesis and collagen deposition. Additionally, blood vessel accumulation in the healing tissue from mice was analyzed by immunofluorescent staining of wound sample cryosections. A motorized tensiometer was also used to assess wound-breaking strength.
Single-cell RNA sequencing data from previous studies were also used to analyze the gene ontology of the fibroblast populations. The role of MFAP5 in fibroblast function was evaluated by treating normal dermal fibroblasts with recombinant MFAP5, with characteristics such as cell migration, proliferation, extracellular matrix-related gene expression, and contractive activity subsequently analyzed. Additionally, the impact of the fibrotic environment on MFAP5 production was evaluated by treating fibroblasts with TGF-β.
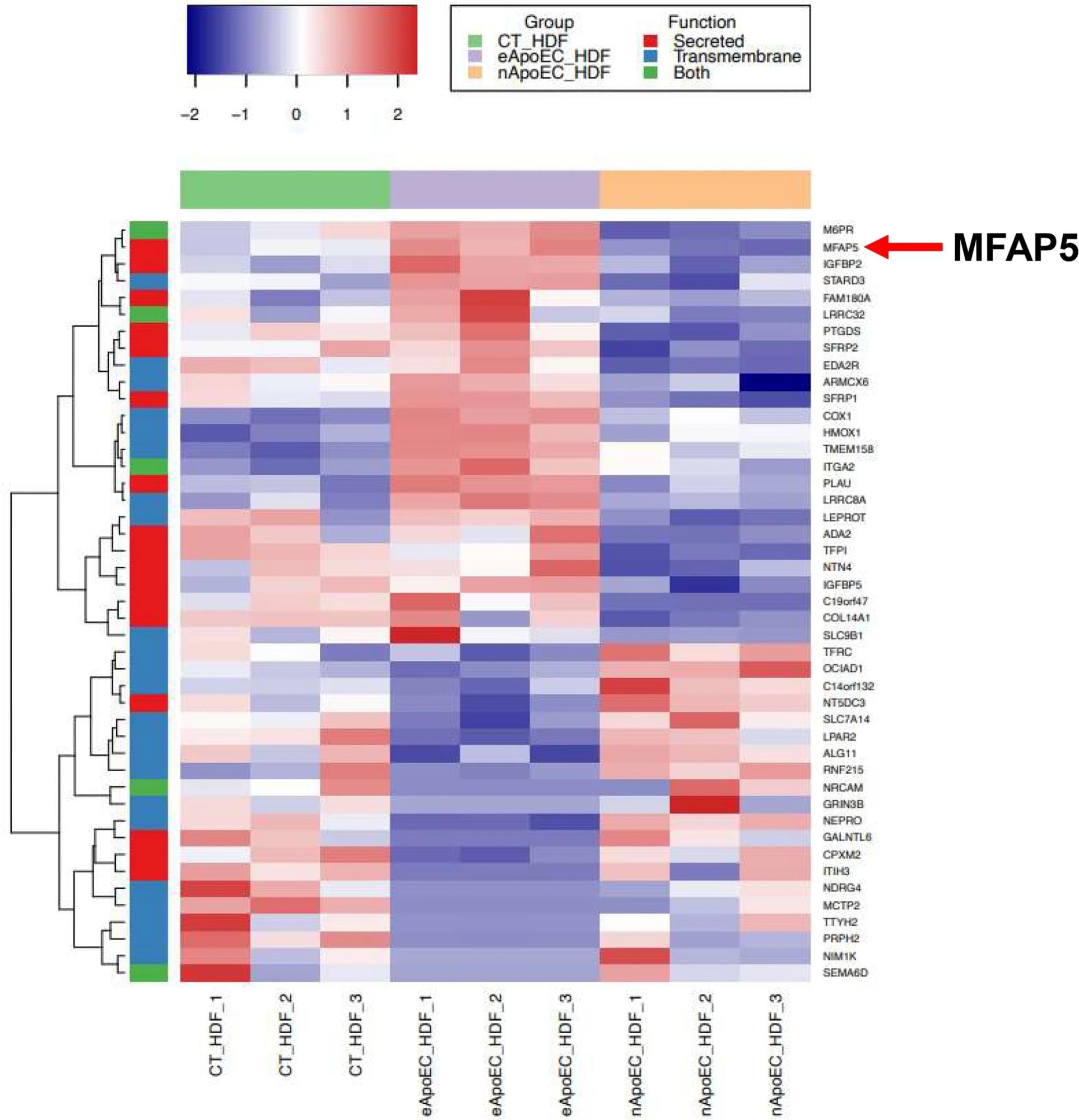 MFAP5 is upregulated in phagocytic fibroblasts. Heatmap of differentially expressed genes between phagocytic and non-phagocytic fibroblasts incubated with GFP-HDMECs (padj ≤ 0.05) that were identified as putative secreted proteins (red), transmembrane proteins (blue) or both (green). Genes were hierarchically clustered using the complete agglomeration method. Each row represents a gene, and each column represents an individual sample in the following groups: control fibroblasts (CT_HDF), fibroblasts phagocytosing apoptotic endothelial cells (eApoEC_HDF), and non-phagocytosing fibroblasts exposed to apoptotic endothelial cells (nApoEC_HDF). N = 3 for each group. The data used was log2 transformed count data. Adjusted p-values were calculated with a Wald test.
MFAP5 is upregulated in phagocytic fibroblasts. Heatmap of differentially expressed genes between phagocytic and non-phagocytic fibroblasts incubated with GFP-HDMECs (padj ≤ 0.05) that were identified as putative secreted proteins (red), transmembrane proteins (blue) or both (green). Genes were hierarchically clustered using the complete agglomeration method. Each row represents a gene, and each column represents an individual sample in the following groups: control fibroblasts (CT_HDF), fibroblasts phagocytosing apoptotic endothelial cells (eApoEC_HDF), and non-phagocytosing fibroblasts exposed to apoptotic endothelial cells (nApoEC_HDF). N = 3 for each group. The data used was log2 transformed count data. Adjusted p-values were calculated with a Wald test.
MFAP5 improves the efficiency of wound healing
MFAP5 expression was significantly high during the remodeling and proliferation phases of wound healing. Moreover, single-cell RNA sequencing data identified two major fibroblast subpopulations involved in MFAP5 expression during wound healing.
When MFAP5 was neutralized by anti-MFAP5 antibodies, wound closure was unaffected. Nevertheless, collagen deposition and angiogenesis levels were lower as compared to controls.
The in vitro experiments using cultured fibroblasts showed that the recombinant MFAP5 substantially enhanced collagen contractility, migration of dermal fibroblasts, and the expression of the extracellular matrix-related pro-fibrotic genes. Additionally, the treatment of dermal fibroblasts with TGF-β significantly increased the expression and production of MFAP5.
Immunofluorescent staining showed that MFAP5 expression was present in the epidermal and dermal layers in normal skin, wound tissue, and keloid tissue. Furthermore, the epidermal staining of MFAP5 was prominent, thus suggesting the involvement of keratinocytes in the production of MFAP5.
Conclusions
The current study evaluated the role of MFAP5 in scar formation by examining its involvement in angiogenesis and collagen deposition and its pro-fibrotic effects.
To this end, MFAP-5 expression increases during the proliferation and remodeling stages of wound healing, with two subpopulations of fibroblasts found to be the major producers of MFAP5. Although wound closure can occur in the absence of MFAP5, angiogenesis levels and collagen deposition are significantly reduced without MFAP5 expression.
Journal reference:
- Han, C., Leonardo, T. R., Romana-Souza, B. et al. (2023). Microfibril-associated protein 5 and the regulation of skin scar formation. Scientific Reports 13(8728). doi:10.1038/s41598-023-35558-x