Spider silk fibers are mostly made up of spidroins, which are a group of large, structural proteins (Figure 1).
Thanks to their elasticity and mechanical strength, the fibers have been effectively used for the rejuvenation of peripheral nerves in rats [1]. It was only recently that the manufacture of these proteins became feasible on an industrial level.

Figure 1. Spider Silk is well known for its extraordinary mechanical properties.
Sweden-based Spiber Technologies aims at marketing recombinant spider proteins, providing customers with customized spidroins for their specific requirements. Demand for high tensile strength has led to the evolution of spidroins, but tensile strength does not always ensure stability whenever other types of stresses are applied.
Here, a Zetasizer Nano ZSP was used to define the stability of three different spidroins at different temperatures, and Malvern Panalytical-patented non-invasive back-scatter (NIBS) technology was used for making all DLS measurements at a scattering detection angle of 173°C.
Results
The results of the cumulants analysis on all three samples at three concentrations are shown in Table 1.
Table 1. Results of cumulants analysis on all three Spidroin constructs at different Concentrations
|
Spidroin Construct
|
Concentration
|
Z-Average Diameter (nm)
|
|
Spidroin 1
|
Conc. 1
|
9.7 ± 0.1
|
|
2 x (Conc. 1)
|
10.8 ± 0.3
|
|
6 x (Conc. 1)
|
13.3 ± 0.6
|
|
Spidroin 2
|
Conc. 1
|
42.0 ± 0.2
|
|
2 x (Conc. 1)
|
47.3 ± 0.1
|
|
6 x (Conc. 1)
|
60.8 ± 0.9
|
|
Spidroin 3
|
Conc. 1
|
14.6 ± 0.8
|
|
2 x (Conc. 1)
|
13.9 ± 0.4
|
|
6 x (Conc. 1)
|
19.7 ± 0.6
|
These results show that Spidroin 2 forms large aggregates even prior to stability trials. The intensity size distributions acquired for Spidroins 1 and 3, pre- and post-storage for one week, are shown in Figure 2. Under these conditions, Spidroin 1 had no visible effect on the sample’s intensity distribution, but significant aggregation was clearly seen in Spidroin 3.
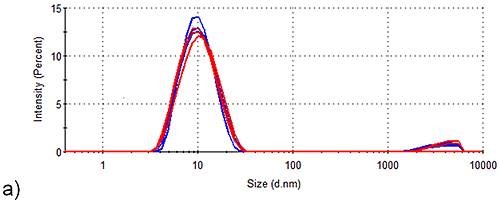
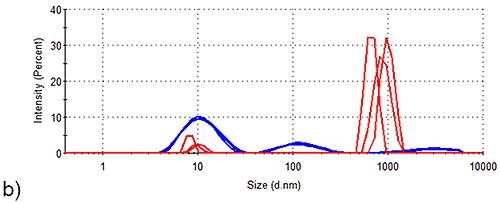
Figure 2. Stability of Spidroin 1 and 3 formulations before and after storage for 1 week. Blue, intensity size distribution prior to storage; Red, intensity size distribution after storage. (a) Spidroin 1 (b) Spidroin 3.
In Table 2, it is shown that aggregates are responsible for 5% of the overall protein-occupied volume of Spidroin 3 after storage.
Table 2. Volume size distribution data of Spidroin 1 and 3 formulations before and after storage for 1 week
|
Sample
|
% Monomer
|
% Aggregate
|
|
Pre-storage Spidroin 1
|
100.0 ± 0.0
|
0.0 ± 0.0
|
|
Post-storage Spidroin 1
|
100.0 ± 0.0
|
0.0 ± 0.0
|
|
Pre-storage Spidroin 3
|
99.9 ± 0.0
|
0.1 ± 0.0
|
|
Post-storage Spidroin 3
|
95.3 ± 3.0
|
4.7 ± 3.0
|
The above data analyses show that Spidroin 1 is the most stable of all the proteins tested. It is seen that before storage, large aggregates already exist in the highly concentrated Spidroin 1 sample. Thanks to the high sensitivity of the Zetasizer Nano ZSP, the kinetics of protein aggregation mechanisms occurring over shorter time periods is evaluated.
Conclusion
Silk proteins are expected to have a high tensile strength. As a result, complete characterization is carried out on their lasting stability in solution. The Zetasizer Nano ZSP was used to identify and precisely size such volume distributions. The impact of concentration on stability was further examined by tracking the aggregation of Spidroin 1 at varied concentrations in the Zetasizer Peltier temperature controlled cell.
References
- Allmeling, C., Jokuszies, A., Reimers, K., Kall, S., Choi, C. Y., Brandes, G., Kasper, C., Scheper, T., Guggenheim, M. & Vogt, P. M. (2008) Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Proliferation 41, 408-420
- Hardy, J. G., Leal-Egaña, A., Scheibel, T. R. (2013) Engineered spider silk proteinbased composites for drug delivery. Macromol. Biosci.13, 1431-1437
- Rising, A., Widhe, M., Johansson, J., Hedhammar, M. (2011) Spider silk proteins: recent advances in recombinant production, structure-function relationships and biomedical applications. Cell. Mol. Life Sci. 68, 169-184
- Tokareva, O, Jacobsen, M., Buehler, M., Wong, J., Kaplan, D. L. (2014) Structurefunction-property-design interplay in biopolymers: Spider silk. Acta Biomater. 10, 1612-1626
About Malvern Panalytical

Malvern Panalytical provides the materials and biophysical characterization technology and expertise that enable scientists and engineers to understand and control the properties of dispersed systems.
These systems range from proteins and polymers in solution, particle and nanoparticle suspensions and emulsions, through to sprays and aerosols, industrial bulk powders and high concentration slurries.
Used at all stages of research, development and manufacturing, Malvern Panalytical’s materials characterization instruments provide critical information that helps accelerate research and product development, enhance and maintain product quality and optimize process efficiency.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.