Governmental agencies such as the EP and FDA impose stringent guidelines to ensure the quality of research, development and production of pharmaceuticals, food substances and other materials on which modern society depends.
Wyatt technology is highly confident that the life-enhancing drug bought tomorrow will be the same as the one bought yesterday and will have a similar therapeutic effect and side effects. These quality assurances benefit the entire society.
Value vs. Waste
One of the greatest endeavors of the modern period is science. Searching and understanding the natural world from quarks to quasars and everything in between, science satisfies humans’ curiosity and enlarges their conscience, and it also improves their material lives with technology that eradicates disease and drudgery. Precisely, society invests vast amounts of money and human capital into the scientific enterprise.
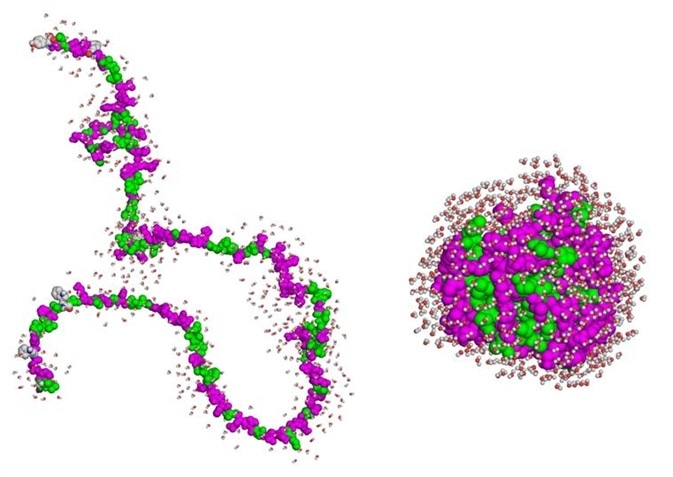
Figure 1. Denatured proteins may remain soluble yet be incompetent to interact
But how much of that capital is wasted? The author Daniel Some is not referring to the pursuit of seemingly uninteresting or useless research, because most often one cannot expect the value of directions of research, or even assign value to them. Neither is the author referring to duplication of effort, which is usually a good thing despite every researchers1 wish to create a novel, breakthrough science. Rather, he refers to good-faith, well-supported lines of research leading to efforts wasted owing to lack of reproducibility, which is a direct result of inadequate quality control of source materials and protocols.
Lack of reproducibility has been at the core of recent discussion in the life sciences community. The journal Nature dedicated a special issue and web page to this topic: see https://www.nature.com/news/reproducibility-1.17552. Concerns about reproducibility and rigor have emerged in several fields of the life sciences, and the community has been addressing it on many fronts from ethics to education. The establishment of well-defined guidelines for rigor, even if executed on a voluntary basis, would form a major step in the right direction to reduce wasted monetary and human capital in this field.
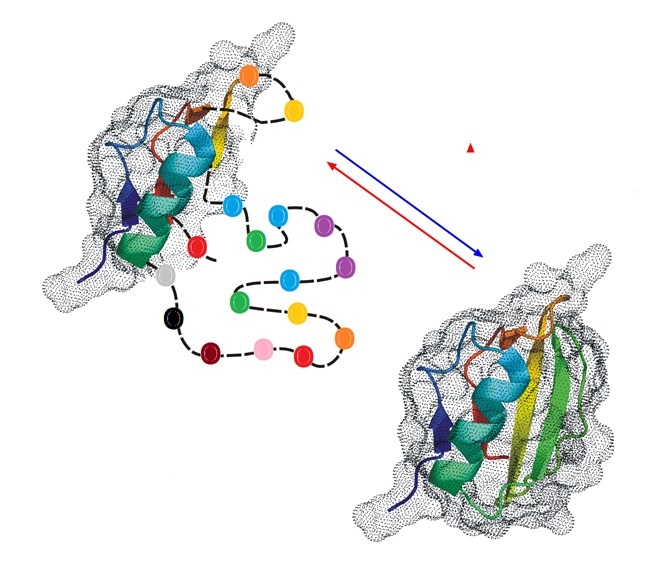
Figure 2. Partial unfolding or inherent disorder may interfere with biological activity
Measure Twice, Publish Once
In this spirit, the Protein Production and Purification Partnership in Europe (P4EU) has acknowledged the need to create guidelines for the verification and characterization of recombinant proteins utilized in research:
Protein Production and Purification Partnership in Europe
There is increasing awareness in the scientific community about the lack of reproducibility and reliability of results published with purified proteins. While protein production is highly regulated and controlled in the pharmaceutical industry by the authorities, there are up to date no guidelines or standards in place in the academic research to guarantee the quality of proteins included in scientific experiments. There is an urgent need to define guidelines for the scientists but also for editors and reviewers of scientific journals and funding agencies for their review processes.
(P4EU web site, https://p4eu.org/protein-quality-standard-pqs, accessed 1/25/2018))
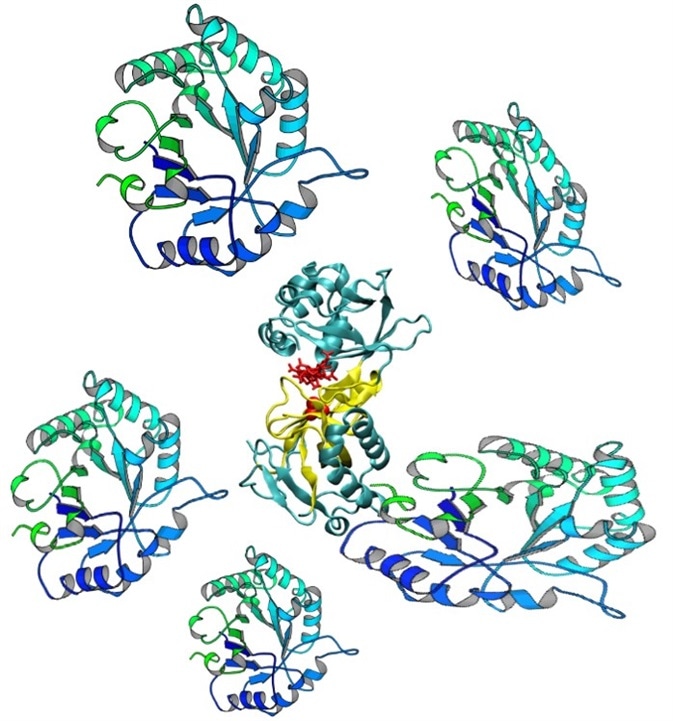
Figure 3. Protein impurities may produce false binding signals
Together with the Association of Resources for Biophysical Research in Europe (ARBRE MOBIEU), P4EU has assembled a team of experts to create a Minimum Protein Quality Standard with an aim to improve the reproducibility and reliability of protein-based research.
The PQS draft is published on the web at https://p4eu.org/protein-quality-standard-pqs. It institutes clear and simple recommendations for the minimum set of biophysical assays that should be carried out on every such protein to guarantee its suitability for use as in experiments, and demands the results to be published in every peer-reviewed paper elucidating such experiments. A few examples of the assays listed include aggregation testing by size-exclusion chromatography with multi-angle light scattering (SEC-MALS) and/or dynamic light scattering (DLS), conformation/folding analysis by circular dichroism (CD), and purity testing by capillary electrophoresis (CE) or SDS-PAGE. The proposed standard contains recommendations for minimum additional information on the production and handling of the proteins to be included in publications, for example concentration (by UV absorbance at 280 nm or otherwise) and a record of storage conditions.
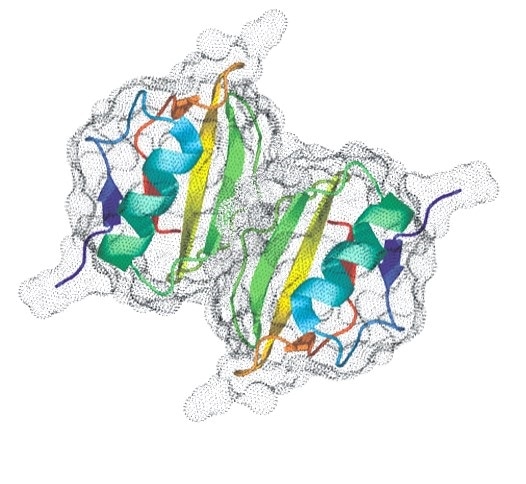
Figure 4. Establishing the Native Oligomeric State is Essential to Understanding Activity
Wyatt Technology acknowledges and supports the establishment of this PQS, and is happy to note that its products such as the miniDAWN, DAWN, and µDAWN MALS detectors and the DynaPro NanoStar DLS detector are important components in protein characterization laboratories worldwide, employed by many members of the team that developed the PQS. Light scattering covers many areas of biophysical characterization such as molar mass, charge, interactions, size, conjugation and conformation, and is applicable from proteins to peptides to exosomes and much more.

Adopt a Standard Today!
Although more careful scientists will usually go beyond the PQS guidelines with regards to quality control assays and reporting, a number of biologists are probably unaware of the need for this degree of quality control. The author believes that the simple existence and publication of the PQS will slowly result in its adoption and implementation, both for the overall benefit of the scientific community and the public at large. The adoption process could be greatly accelerated if journal reviewers and editors took note and started recommending or demanding the inclusion of the PQS data in publications. The adoption of the PQS by funding agencies such as the NSF and NIH and NSF might be even more relevant for reducing the waste of public funds and for increasing confidence in government management of research.
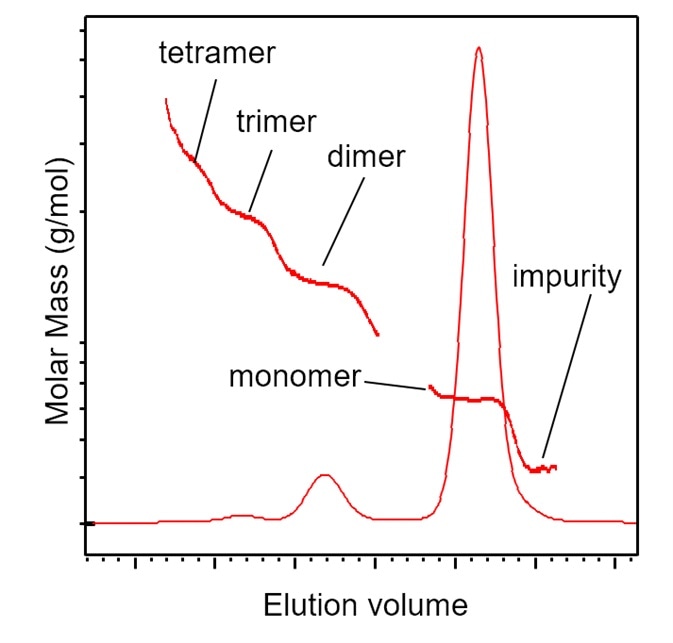
Figure 5. SEC-MALS identification of aggregates and impurities
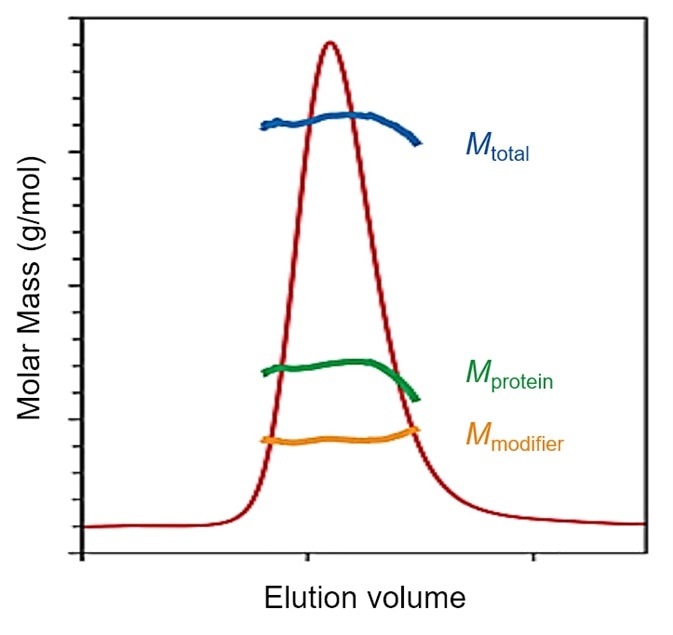
Figure 6. SEC-MALS determination of glycosylation or membrane protein loading of lipids
References
- Quality assessment and optimization of purified protein samples: why and how? Raynal et al. Microbial Cell Factories 13:180 (2014)
- The Trip Adviser guide to the protein science world: a proposal to improve the awareness concerning the quality of recombinant proteins. Mario Lebendiker, Tsafi Danieli and Ario de Marco, BMC Res Notes. 7:585 (2014 Sep 1).
- Reagent validation: an underestimated issue in laboratory practice. Ario de Marco. J. Mol. Recognit. 23, 136 (2010)
- Recombinant protein quality evaluation: proposal for a minimal information standard. Buckle, A.M et al. Standards in Genomic Sciences 5, 195-197 (2011)
- Daviter T, Fronzes R: Protein Sample Characterization. In Protein-Ligand Interactions: Methods and Applications. Edited by Williams MA, Daviter T. Totowa, NJ: Humana Press; 35–62 Methods in Molecular Biology, vol 1008 (2013).
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.