It is an extremely tedious task to identify new small-molecule drugs through high-throughput screening (HTS). As a result, engineers and scientists have collaborated to automate the entire process of trudging through indefinite number of compounds, evaluating their binding to proteins of interest and searching for the few precious compounds that indeed bind.
However, most often, those ‘hits’ occur one in ten thousand compounds or even one in a million compounds, and also considerable effort has to be made to further ensure that these compounds are not ‘false positives’― that is they indeed bind. Then, finally, it has to be established whether therapeutic effect results from their interaction.
Getting a true hit is a far-fetched scenario, giving way to bogus mechanisms and creating false positives, which possess many of the characteristics of binding to the protein of interest but ultimately lead to a dead end in terms of valuable drugs.
‘Promiscuous inhibitors’ are referred to a general source of false positives and are defined as compounds which despite having no therapeutic effect seem to prevent a wide range of enzymes in HTS. At the UCSF Department of Pharmaceutical Chemistry, Professor Brian Shoichet heads the leading lab that is studying the mechanisms of promiscuous inhibitors.
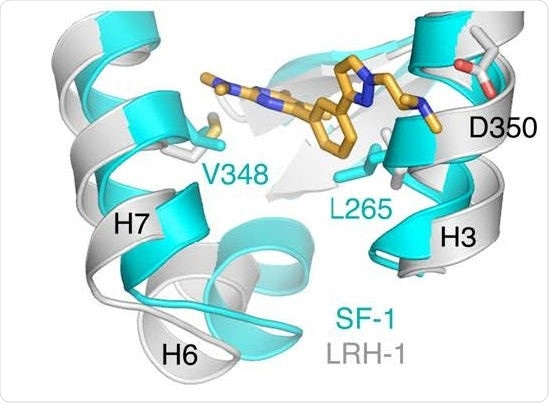
Co-crystallization structure of a small molecule inhibitor bound to enzyme. Benod et al. 2013
Finding a false-positive culprit: colloidal aggregates
Shoichet and his colleagues observed that promiscuous inhibition mostly occurs when the compound aggregates into colloidal particles with sizes ranging from tens to hundreds of nm (McGovern et al., J. Med. Chem. 2003, 46, 4265-4272; Seidler et al., J. Med. Chem. 2003, 46, 4477-4486).
These particles can sequester proteins via surface adsorption, but these also have highly non-specific interactions with proteins: in an assay, the particulates can strongly prevent an enzyme, but when another protein such as serum albumin is added it readily displaces the enzymes, which can freely continue their activity.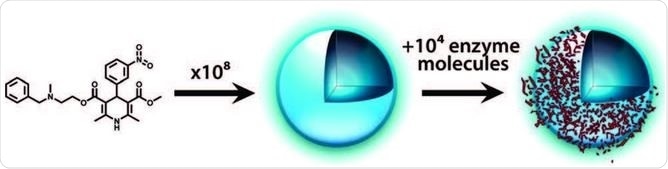
Process of promiscuous inhibition: compound aggregates into colloidal particles that then sequester enzyme on their surface. Coan and Shoichet 2008.
The inhibitory effect also seems to be reduced when higher amounts of enzyme are added. This is due to the saturation of particulate surfaces and hence there is surplus enzyme to act upon the substrate. Most of this behavior is explained in Shoichet’s 2006 Drug Discovery Today article that is interestingly titled as “Screening in a spirit haunted world”.
Colloidal aggregate formation is believed to occur in a highly concentration-dependent way (Coan and Shoichet, J. Am. Chem. Soc. 2008, 130, 9606–9612), and according to the research team this behavior is responsible for the unusual response curves, i.e., the “bell-shaped” curve, shown by some compounds: the drug seems to have a high effect at low dose, but loses its efficacy at higher concentrations (Owen et al., ACS Chem. Biol. 2014, 9(3), 777–784).
At the critical aggregation concentration (CAC), this loss of efficacy closely correlates with the initiation of aggregation.
Dynamic Light Scattering helps lose those colloidal aggregates
Using DynaPro cuvette-based DLS detector, dynamic light scattering or DLS measurements were made which helped explain the role of colloidal aggregate formation for promiscuous inhibitors and also helped determine the size of particles that were formed together with their CAC.
Although conducting the fundamental research into the mechanism and cause of promiscuous inhibition is good, what is really important is the fact that compounds which aggregate and show robust potential for promiscuous inhibition should be screened out. This will inhibit the appearance of these compounds as false positives in HTS.
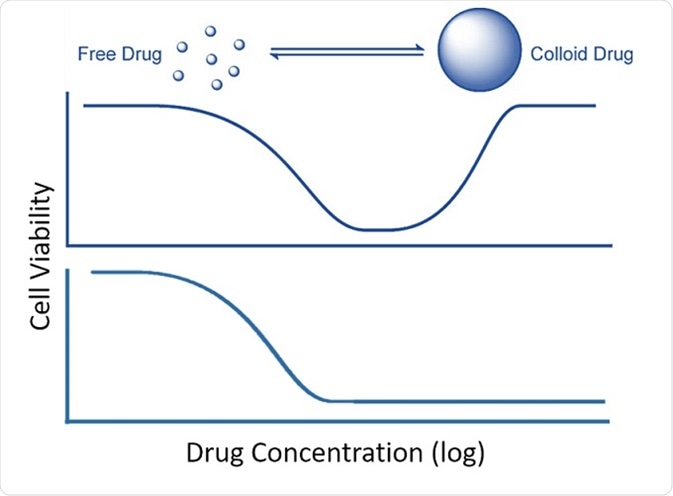
Effect of colloidal aggrege formation on inhibition. Top: bell-shaped curve resulting from colloidal aggregation. Bottom: standard inhibition curve. After Owen et al., 2014
A number of screening methods have been developed by Shoichet lab of which, one of which, depends on the DynaPro Plate Reader to detect aggregation (Feng et al., Nat. Chem. Biol. 2005, 1, 146 – 148).
“The DynaPro instrument is our first line of defense for checking whether a group of molecules are acting as aggregators."
Professor Brian Shoichet, UCSF Department of Pharmaceutical Chemistry.
It has now becoming a standard practice to test a lead compound for aggregation so that all false positives can be easily eliminated. Although most of the recent studies performed at the Shoichet lab involve the simulations of in silico docking— increasing the virtual screening space and simultaneously decreasing the number of required physical assays— aggregation testing continues to be a regular operating proctor (Carlsson et al, J. Med. Chem. 2010, 53, 3748–3755; Benod et al., J. Biol. Chem. 2013, 288, 19830-19844).
In addition, drug cocktails are attracting a great deal of interest, like those used for treating HIV with broad-spectrum antibiotics, and as a result it is very crucial to confirm that aggregation is not cross-induced by non-aggregating compounds (Cokal et al., Chem. & Biol. 2014, 21, 541–551.)

The DynaPro DLS Plate Reader II
If at all a compound aggregates, it is not a complete loss. By identifying ideal conditions, which mostly involve a low detergent concentration, the compound can be kept monomeric and a more consistent test can be carried out for specific inhibition.
“The 384-well format makes looking for particle formation at multiple concentration ranges, and multiple conditions (e.g., with and without detergent), very convenient."
Professor Brian Shoichet, UCSF Department of Pharmaceutical Chemistry.
Finding beneficial aggregation
As such, small-molecule drugs can benefit from aggregation. Analyses of the drugs’ colloidal behavior in conditions simulating intestinal fluid suggest that bioavailability upon aggregation can be considerably increased by orally-dosed drugs, making the so-called low-efficacy compounds to be actually effective (Doak et al., J. Med. Chem. 2010, 53, 4259–4265).
Here, critical input was also provided by the DLS technique to establish the conditions of aggregation, thus helping to spot the immense advantages of colloid formation.
Acknowledgements
Produced from materials originally authored by Daniel Some, Ph.D. | Principal Scientist, Wyatt Technology Corp.
About Wyatt
 With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
With a long history of excellence in scientific instrumentation, Wyatt Technology is the recognized leader in innovative light scattering instruments, accessories, software and services for determining the properties of macromolecules and nanoparticles in solution. Wyatt provides cutting-edge solutions for in-line multi-angle static light scattering (SEC-MALS), field-flow fractionation (FFF-MALS), composition gradients (CG-MALS), high-throughput and traditional dynamic light scattering (DLS), electrophoretic mobility via phase-analysis light scattering (MP-PALS), differential refractometry and differential viscosity. With a staff composed of 20% Ph.D. scientists and many more dedicated and experienced support personnel, Wyatt's aim is to delight the customer with the best products, training, customer support and service available in the industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.