In the last ten years, chimeric antigen receptor T cells (CAR-T cells) have become the dominant driver for growth in the field of cell and gene therapy (CGT) development. The history and potential future of CAR-T cells are reviewed in this article.
Image Credit: BioIVT
T cells and cancer
T cells are vital for surveillance within the immune system. They are activated once the T cell receptor (TCR) binds its cognate peptide found in cell surface proteins encoded by the major histocompatibility complex (MHC) gene family on an antigen-presenting cell (APC).
The recognition of both an MHC and peptide makes TCR binding more complicated than the simple binding of a B cell antibody to an antigen. After a TCR binds to its cognate peptide MHC, the T cell needs a second signal (via costimulatory receptors) to activate fully.
Activated T cells then induce a cytokine release and a series of proliferation and differentiation begins. The receptiveness of T cells is thus limited in a way that B cells are not—T cells only respond to peptides that have been processed and presented on native MHCs and necessitate a second signal for full activation.
Extracellular proteins, bacteria, or viruses presented without processing, or those in a foreign MHC complex, do not provoke an immune response. The absence of a costimulatory signal results in T cell anergy.
T cells’ ability to detect non-self-antigens and initiate an immune response has prompted researchers to investigate whether T cells can detect and eliminate tumor cells.
Evasion is accomplished through various mechanisms. Cancers may fail to provoke an immune response as there are no non-self-peptides or proteins presented to B and T cells. Cancer cells do, however, upregulate the expression of some stress-related genes that trigger a T-cell response.
If a response does not eliminate the malignant cells, the surviving cells may then avoid immune detection (either by downregulating MHC protein expression on the surface or by slowing the proteolytic process that produces peptides for display in MHC proteins).
Through mouse models involving cancer cells contrived to present a foreign peptide/protein on the cell surface, researchers have shown that the immune system can efficiently destroy cancer cells if it can recognize them as non-self-cells, and have begun to explore methods to activate a similar response in humans.
CARs
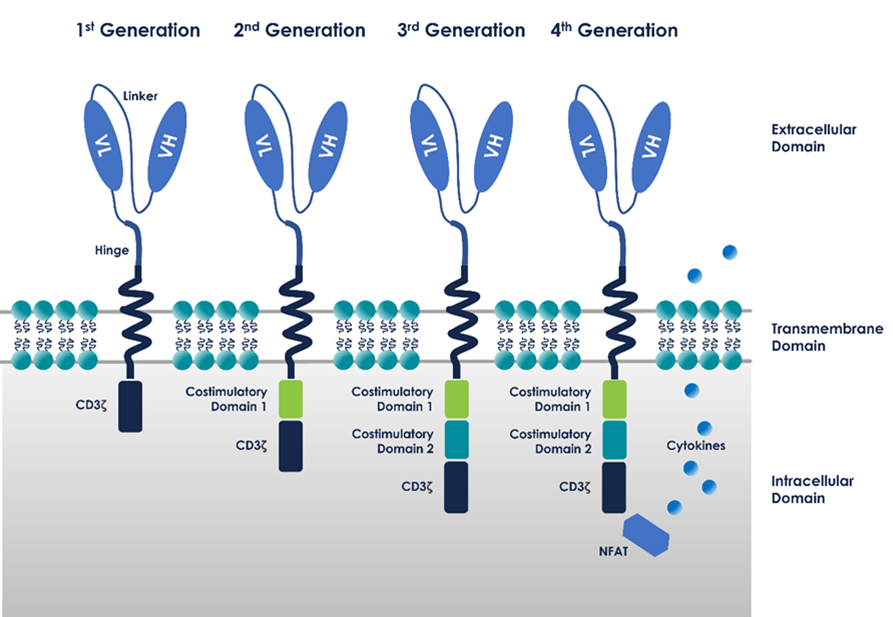
Chimeric antigen receptors (CARs), target specific and generally native antigens. The first-generation CAR-T cells were made of a single-chain variable fragment (scFv) from an antibody that targeted the antigen of interest, fused with the activating domains of CD3ζ or Fc receptor γ.
These early CARs triggered a cytolytic response but could not produce cytokines or expand as they lacked a secondary activation signal.2 Later generations of CAR design used costimulatory molecules alongside activating domains to produce a full series of T-cell responses.
These second-generation CARs are made of the CD3ζ activation domain fused to the cytoplasmic domain of a costimulatory molecule, like CD28, OX40, DAP10, 4-1BB, or ICOS. These cells are adept in cytokine secretion and sustained expansion in the face of repeated exposure to antigens.3
Third-generation CARs use a “triple-decker” structure which adds the activating domain of CD3ζ to two costimulatory receptor cytoplasmic domains.
Fourth-generation CARs—“T cell redirected for universal cytokine killing” (TRUCK) or “armored CARs”—merge the properties of second-generation CARs with an enhanced efficiency against tumors, including a greater capability of cytokine secretion.4
Elahi, Reza, et al. (2018) Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells. Front. Immunol. DOI: 10.3389/fimmu.2018.01717.
CAR-T cell production
The method of manufacturing CAR-T cells follows a high-level and canonical process. Via leukapheresis, cells are collected. The patient provides cells for autologous therapies, whereas healthy donors provide them for allogeneic therapies.
By washing the material and performing red blood cell (RBC) lysis and platelet removal, leukopaks are processed. This is particularly important when studying the impact of RBC contaminants on cell cultures downstream.
T cells are then isolated through immunomagnetic separation. T-cell activation is frequently used in conjunction with the isolation step by using anti-CD3 and anti-CD28 selection to streamline workflows.
After isolation and activation, T cells are primed for transformation into CAR-T cells. Historically, this was achieved through either lentiviral vector (LVV) or gammaretroviral vector (GRVV), since both can sustain relatively large genomic payloads and integrate into the T cell genome.5
More recently, alternative methods of CAR-T cell transformation have been explored, including Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and non-viral electroporation methods.
After the successful transformation of T cells, CAR-T cells are expanded in culture to reach the appropriate therapeutic dose. This is dependent on the therapy and can be millions of cells per kilogram of the patient’s body weight. The culture volume scales accordingly; thus, CAR-T cells must be harvested and resuspended to injectable volumes.
After harvesting, the CAR-T cells are washed again and resuspended in their final formulation, often with a cryopreservation agent necessary for the protection of the cells during transport from the manufacturing site to the patient.
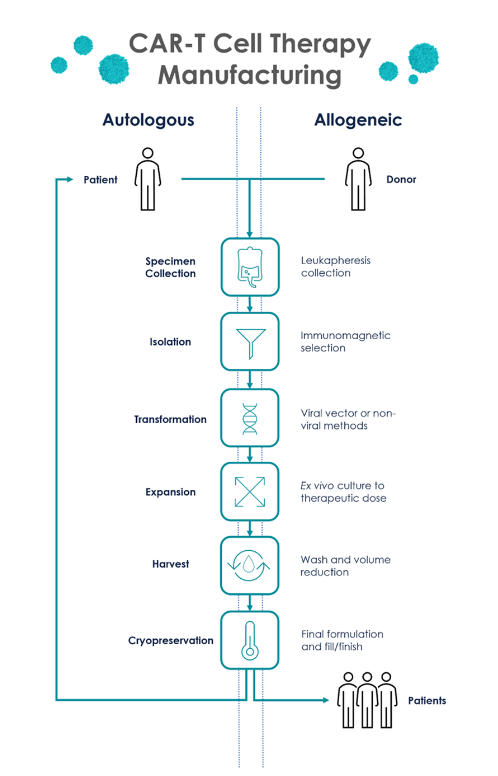
Image Credit: BioIVT
Advancements in CAR-T cells
Researchers persist in advancing CAR-T cell technology, endeavoring to increase the number of patients treated per lot and to shorten or eradicate the manufacturing process.
An important focus area is the clinical advancement of allogeneic CAR-T cell therapies. The most cost-effective therapies are allogeneic, as CAR-T cells can be used to treat multiple patients, decreasing the cost per dose. The so-called Sleeping Beauty-engineered CAR-T cells use healthy donor CD34+ hematopoietic stem cells (HSCs) and transformation through plasmid technologies.6
Another innovation is the identification of ultra-specific T-cell phenotypes to maximize the efficacy of transformation and minimize manufacturing times. An example of this is Novartis’s T-Charge CAR-T platform, which targets the preservation of the naïve and stem cell memory T cell (Tscm) populations to transfer CAR-T cell production back into the patient’s own body following injection. This decreases manufacturing times from several weeks to 24 hours.7
Other technologies can even eliminate the ex vivo manipulation of T cells. Studies on mice have shown that an adeno-associated virus (AAV) containing a CAR gene can be injected into the in vivo environment to convert T cells into CAR-T cells.8 While still in the early development stages, this method could shift CAR-T research further into viral vector manufacturing.
Conclusion
CAR-T cell research is an innovative field. Its therapeutic effectiveness has been established via unmitigated patient remission rates, and new technologies constantly seek to advance the field further.
Researchers may want to utilize starting materials that most accurately reflect the final therapeutic workflow, including oncology patient-collected PBMCs for autologous applications, and cryopreserved or fresh research-grade leukopaks for early research and development.
Researchers may utilize human AB serum supplementation for their culture media to encourage cellular proliferation and reach therapeutic doses for any work that requires expansion.
References and further reading
- Swann, J. and Smyth, M. (2007) Immune Surveillance of Tumors. J Clin Invest. 117(5), pp.1137-1146. https://pubmed.ncbi.nlm.nih.gov/17476343/
- Sadelain, M. et al. (2013) The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 3(4), pp.388-398. https://pubmed.ncbi.nlm.nih.gov/23550147/
- Krause, A. et al. (1998) Antigen-Dependent CD28 Signaling Selectively Enhances Survival and Proliferation in Genetically Modified Activated Human Primary T Lymphocytes. J Exp Med. 188(4), pp.619-626. https://pubmed.ncbi.nlm.nih.gov/9705944/
- Elahi, R. et al. (2018) Immune Cell Hacking: Challenges and Clinical Approaches to Create Smarter Generations of Chimeric Antigen Receptor T Cells. Front Immunol. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6080612/
- Sengupta, R. (2021) Gammaretroviral and Lentiviral Vector Manufacture: Brief Overview. Cell & Gene Therapy Insights. 7(3), pp.345-354. https://www.insights.bio/cell-and-gene-therapy-insights/journal/article/2012/Gammaretroviral-and-lentiviral-vector-manufacture-brief-overview?msclkid=03ac9e87cfd511ec8aa302872078cf0b
- Magnani, C. et al. (2020) Sleeping Beauty–Engineered CAR T Cells Achieve Antileukemic Activity Without Severe Toxicities. J Clin Invest. 130(11), pp.6021-6033. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7598053/
- Engels, B et al. (2021) Preservation of T-Cell Stemness with a Novel Expansionless CAR-T Manufacturing Process, Which Reduces Manufacturing Time to Less Than Two Days, Drives Enhanced CAR-T Cell Efficacy. ASH Annual Meeting & Exposition. https://ash.confex.com/ash/2021/webprogram/Paper146246.html
- Nawaz, W et al. (2021) AAV-Mediated In Vivo CAR Gene Therapy for Targeting Human T-Cell Leukemia. Blood Cancer Journal. 11(119). https://www.nature.com/articles/s41408-021-00508-1
About BioIVT
BioIVT, formerly BioreclamationIVT, is a leading global provider of high-quality biological specimens and value-added services. We specialize in control and disease state samples including human and animal tissues, cell products, blood, and other biofluids. Our unmatched portfolio of clinical specimens directly supports precision medicine research and the effort to improve patient outcomes by coupling comprehensive clinical data with donor samples.
Our Research Services team works collaboratively with clients to provide in vitro hepatic modeling solutions. And as the world’s premier supplier of ADME-Tox model systems, including hepatocytes and subcellular fractions, BioIVT enables scientists to better understand the pharmacokinetics and drug metabolism of newly discovered compounds and the effects on disease processes. By combining our technical expertise, exceptional customer service, and unparalleled access to biological specimens, BioIVT serves the research community as a trusted partner in ELEVATING SCIENCE®.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.